
Back إعادة تعريف الوحدات الأساسية في النظام الدولي للوحدات Arabic Новыя вызначэнні СІ Byelorussian Новите определения в SI Bulgarian Nové definice SI Czech СИ пĕрчисен палăртавĕсен улшăнăвĕсем (2019) CV 2019 redefinition af SI-enhederne Danish Redefinición de las unidades del SI Spanish 2019ko SI oinarrizko unitateen birdefinizioa Basque بازتعریف یکاهای پایه اسآی (۲۰۱۹) Persian Redéfinition du Système international d'unités de 2018-2019 French
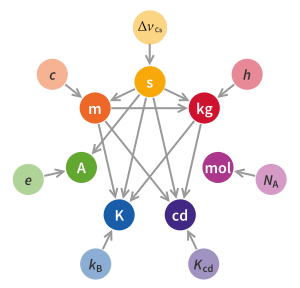

In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artefacts such as the standard kilogram.[1][2] Effective 20 May 2019, the 144th anniversary of the Metre Convention, the kilogram, ampere, kelvin, and mole are now defined by setting exact numerical values, when expressed in SI units, for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (kB), and the Avogadro constant (NA), respectively. The second, metre, and candela had previously been redefined using physical constants. The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year after determining that previously agreed conditions for the change had been met.[7]: 23 These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.
The previous major change of the metric system occurred in 1960 when the International System of Units (SI) was formally published. At this time the metre was redefined: the definition was changed from the prototype of the metre to a certain number of wavelengths of a spectral line of a krypton-86 radiation, making it derivable from universal natural phenomena.[Note 1] The kilogram remained defined by a physical prototype, leaving it the only artefact upon which the SI unit definitions depend. At this time the SI, as a coherent system, was constructed around seven base units, powers of which were used to construct all other units. With the 2019 redefinition, the SI is constructed around seven defining constants, allowing all units to be constructed directly from these constants. The designation of base units is retained but is no longer essential to define the SI units.[4]
The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts – the prototype of the metre and prototype of the kilogram – when the metric system was introduced in France in 1799. Although they were designed for long-term stability, the prototype kilogram and its secondary copies have shown small variations in mass relative to each other over time; they are not thought to be adequate for the increasing accuracy demanded by science, prompting a search for a suitable replacement. The definitions of some units were defined by measurements that are difficult to precisely realise in a laboratory, such as the kelvin, which was defined in terms of the triple point of water. With the 2019 redefinition, the SI became wholly derivable from natural phenomena with most units being based on fundamental physical constants.
A number of authors have published criticisms of the revised definitions; their criticisms include the premise that the proposal failed to address the impact of breaking the link between the definition of the dalton[Note 2] and the definitions of the kilogram, the mole, and the Avogadro constant.
- ^ "BIPM statement: Information for users about the proposed revision of the SI" (PDF). Archived (PDF) from the original on 21 January 2018. Retrieved 5 May 2018.
- ^ "Decision CIPM/105-13 (October 2016)". Archived from the original on 24 August 2017. Retrieved 31 August 2017.
- ^ Kühne, Michael (22 March 2012). "Redefinition of the SI". Keynote address, ITS9 (Ninth International Temperature Symposium). Los Angeles: NIST. Archived from the original on 18 June 2013. Retrieved 1 March 2012.
- ^ a b "9th edition of the SI Brochure". BIPM. 2019. Retrieved 20 May 2019.
- ^ "Historic Vote Ties Kilogram and Other Units to Natural Constants". NIST. 16 November 2018. Archived from the original on 18 November 2018. Retrieved 16 November 2018.
- ^ Milton, Martin (14 November 2016). Highlights in the work of the BIPM in 2016 (PDF). SIM XXII General Assembly. Montevideo, Uruguay. p. 10. Archived from the original (PDF) on 1 September 2017. Retrieved 13 January 2017. The conference ran from 13–16 November and the vote on the redefinition was scheduled for the last day. Kazakhstan was absent and did not vote.
- ^ Cite error: The named reference
cipm_106was invoked but never defined (see the help page). - ^ Crease, Robert P. (2011). "France: "Realities of Life and Labor"". World in the Balance. New York: W. W. Norton & Company, Inc. pp. 83–84. ISBN 978-0-393-07298-3.
Cite error: There are <ref group=Note> tags on this page, but the references will not show without a {{reflist|group=Note}} template (see the help page).


