
Back مطيافية الامتصاص Arabic Apsorpcijska spektroskopija BS Espectroscòpia d'absorció Catalan Espectroscopia de absorción Spanish Xurgaketa-espektroskopia Basque طیفبینی جذبی Persian Spectrométrie d'absorption French Speictreascópacht ionsúcháin Irish Apsorpcijska spektroskopija Croatian Կլանման սպեկտրալ գիծ Armenian

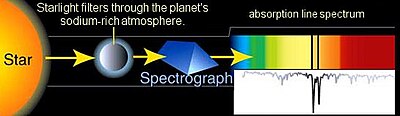
Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.
Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing.
There is a wide range of experimental approaches for measuring absorption spectra. The most common arrangement is to direct a generated beam of radiation at a sample and detect the intensity of the radiation that passes through it. The transmitted energy can be used to calculate the absorption. The source, sample arrangement and detection technique vary significantly depending on the frequency range and the purpose of the experiment.
Following are the major types of absorption spectroscopy:[1]
| Sr. No | Electromagnetic radiation | Spectroscopic type |
|---|---|---|
| 1 | X-ray | X-ray absorption spectroscopy |
| 2 | Ultraviolet–visible | UV–vis absorption spectroscopy |
| 3 | Infrared | IR absorption spectroscopy |
| 4 | Microwave | Microwave absorption spectroscopy |
| 5 | Radio wave | Electron spin resonance spectroscopy |
- ^ Kumar, Pranav (2018). Fundamentals and Techniques of Biophysics and Molecular biology. New Delhi: Pathfinder publication. p. 33. ISBN 978-93-80473-15-4.