Back Asynsuur Afrikaans حمض الخليك Arabic Ácidu acético AST Sirkə turşusu Azerbaijani استیک اسید AZB Asam asétat BAN Asidong asetiko BCL Воцатная кіслата Byelorussian Оцетна киселина Bulgarian অ্যাসিটিক অ্যাসিড Bengali/Bangla
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetic acid[3] | |||
| Systematic IUPAC name
Ethanoic acid | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Abbreviations | AcOH | ||
| 506007 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.528 | ||
| EC Number |
| ||
| E number | E260 (preservatives) | ||
| 1380 | |||
| KEGG | |||
| MeSH | Acetic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2789 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
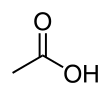
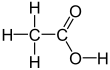
| CH3COOH | |||
| Molar mass | 60.052 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Heavily vinegar-like | ||
| Density | 1.049 g/cm3 (liquid); 1.27 g/cm3 (solid) | ||
| Melting point | 16 to 17 °C; 61 to 62 °F; 289 to 290 K | ||
| Boiling point | 118 to 119 °C; 244 to 246 °F; 391 to 392 K | ||
| Miscible | |||
| log P | −0.28[4] | ||
| Vapor pressure | 1.54653947 kPa (20 °C) 11.6 mmHg (20 °C)[5] | ||
| Acidity (pKa) | 4.756 | ||
| Conjugate base | Acetate | ||
| −31.54·10−6 cm3/mol | |||
Refractive index (nD)
|
1.371 (VD = 18.19) | ||
| Viscosity | 1.22 mPa s 1.22 cP | ||
| 1.74 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
123.1 J/(K⋅mol) | ||
Std molar
entropy (S⦵298) |
158.0 J/(K⋅mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−483.88–483.16 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−875.50–874.82 kJ/mol | ||
| Pharmacology | |||
| G01AD02 (WHO) S02AA10 (WHO) | |||
| Legal status |
| ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H226, H314 | |||
| P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 40 °C (104 °F; 313 K) | ||
| 427 °C (801 °F; 700 K) | |||
| Explosive limits | 4–16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
3.31 g/kg, oral (rat) | ||
LC50 (median concentration)
|
5620 ppm (mouse, 1 h) 16000 ppm (rat, 4 h)[7] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm (25 mg/m3)[6] | ||
REL (Recommended)
|
TWA 10 ppm (25 mg/m3) ST 15 ppm (37 mg/m3)[6] | ||
IDLH (Immediate danger)
|
50 ppm[6] | ||
| Related compounds | |||
Related carboxylic acids
|
Formic acid Propionic acid | ||
Related compounds
|
Acetaldehyde Acetamide Acetic anhydride Chloroacetic acid Acetyl chloride Glycolic acid Ethyl acetate Potassium acetate Sodium acetate Thioacetic acid | ||
| Supplementary data page | |||
| Acetic acid (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Identifiers | |
| E number | E260 (preservatives) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.528 |
| Data page | |
| Acetic acid (data page) | |
Acetic acid /əˈsiːtɪk/, systematically named ethanoic acid /ˌɛθəˈnoʊɪk/, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats.
The global demand for acetic acid as of 2023 is about 17.88 million metric tonnes per year (t/a). Most of the world's acetic acid is produced via the carbonylation of methanol. Its production and subsequent industrial use poses health hazards to workers, including incidental skin damage and chronic respiratory injuries from inhalation.
- ^ Scientific literature reviews on generally recognised as safe (GRAS) food ingredients. National Technical Information Service. 1974. p. 1.
- ^ "Chemistry", volume 5, Encyclopædia Britannica, 1961, page 374.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 745. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ "acetic acid_msds".
- ^ Lange's Handbook of Chemistry, 10th ed.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0002". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Acetic acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).