
Back الفيروسات المرتبطة بالغدية Arabic الفيروسات المرتبطه بالغديه ARZ Virus adenoassociat Catalan Adeno-assoziierte Viren German Virus adenoasociado Spanish Virus adéno-associé French Adeno-associated virus HE Ադենոկախյալ վիրուս Armenian Virus adeno-associati Italian アデノ随伴ウイルス Japanese
| Adeno-associated virus | |
|---|---|
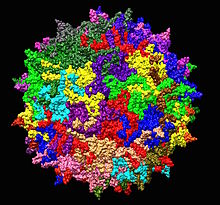
| |
| Adeno-associated virus serotype 2 structure from 1LP3. One fivefold axis shown center. | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Quintoviricetes |
| Order: | Piccovirales |
| Family: | Parvoviridae |
| Subfamily: | Parvovirinae |
| Genus: | Dependoparvovirus |
| Viruses included: | |
| |
Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small (approximately 26 nm in diameter) replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).[1][2]
Several features make AAV an attractive candidate for creating viral vectors for gene therapy, and for the creation of isogenic human disease models.[3] Gene therapy vectors using AAV can infect both dividing and quiescent cells and persist in an extrachromosomal state without integrating into the genome of the host cell. In the native virus, however, integration of virally carried genes into the host genome does occur.[4] Integration can be important for certain applications, but can also have unwanted consequences. Recent human clinical trials using AAV for gene therapy in the retina have shown promise.[5]
In March 2023, a series of Nature papers detected high titres of adeno-associated virus 2 (AAV2), alongside adenovirus and herpesvirus, in samples from a wave of childhood hepatitis.[6] One paper suggested that AAV2 co-infection may contribute to more serious liver disease than infection with only adeno- or herpesviruses and that the causal link remains to be established.[7]
- ^ Naso MF, Tomkowicz B, Perry WL, Strohl WR (August 2017). "Adeno-Associated Virus (AAV) as a Vector for Gene Therapy". BioDrugs. 31 (4): 317–334. doi:10.1007/s40259-017-0234-5. PMC 5548848. PMID 28669112.
- ^ Wu Z, Yang H, Colosi P (January 2010). "Effect of genome size on AAV vector packaging". Molecular Therapy. 18 (1): 80–86. doi:10.1038/mt.2009.255. PMC 2839202. PMID 19904234.
- ^ Grieger JC, Samulski RJ (2005). "Adeno-associated Virus as a Gene Therapy Vector: Vector Development, Production and Clinical Applications". Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Advances in Biochemical Engineering/Biotechnology. Vol. 99. pp. 119–45. doi:10.1007/10_005. ISBN 978-3-540-28404-8. PMID 16568890.
- ^ Deyle DR, Russell DW (August 2009). "Adeno-associated virus vector integration". Current Opinion in Molecular Therapeutics. 11 (4): 442–7. PMC 2929125. PMID 19649989.
- ^ Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. (May 2008). "Safety and efficacy of gene transfer for Leber's congenital amaurosis". The New England Journal of Medicine. 358 (21): 2240–8. doi:10.1056/NEJMoa0802315. PMC 2829748. PMID 18441370.
- ^ Tacke F (May 2023). "Severe hepatitis outbreak in children linked to AAV2 virus". Nature. 617 (7961): 471–472. Bibcode:2023Natur.617..471T. doi:10.1038/d41586-023-00570-8. PMID 36997704.
- ^ Servellita V, Sotomayor Gonzalez A, Lamson DM, Foresythe A, Huh HJ, Bazinet AL, et al. (May 2023). "Adeno-associated virus type 2 in US children with acute severe hepatitis". Nature. 617 (7961): 574–580. Bibcode:2023Natur.617..574S. doi:10.1038/s41586-023-05949-1. PMC 10170441. PMID 36996871.