
Back تآصل الحديد Arabic ئالۆتڕۆپی ئاسن CKB Alotropické modifikace železa Czech Burdin alotropoak Basque دگرشکلیهای آهن Persian Alotropije željeza Croatian Hamskiptarit járns Icelandic Odmiany alotropowe żelaza Polish Alotropije željeza Serbo-Croatian Allotropes of iron SIMPLE
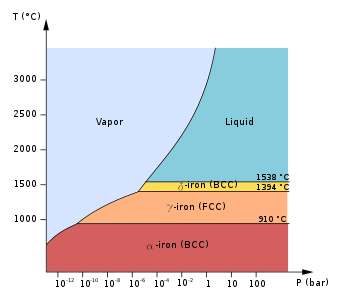

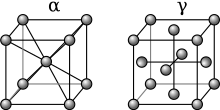
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe, ferrite), gamma iron (γ-Fe, austenite), and delta iron (δ-Fe). At very high pressure, a fourth form exists, epsilon iron (ε-Fe, hexaferrum). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures.[1]
The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure.[2][3][4] The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements.
- ^ Boehler, Reinhard (2000). "High-pressure experiments and the phase diagram of lower mantle and core materials". Reviews of Geophysics. 38 (2). American Geophysical Union: 221–245. Bibcode:2000RvGeo..38..221B. doi:10.1029/1998RG000053. S2CID 33458168.
- ^ Cohen, Ronald E.; Stixrude, Lars. "Crystal at the Center of the Earth". Archived from the original on 5 February 2007. Retrieved 2007-02-05.
- ^ Stixrude, Lars; Cohen, Ronald E. (March 1995). "High-Pressure Elasticity of Iron and Anisotropy of Earth's Inner Core". Science. 267 (5206): 1972–5. Bibcode:1995Sci...267.1972S. doi:10.1126/science.267.5206.1972. PMID 17770110. S2CID 39711239.
- ^ "What is at the centre of the Earth?". BBC News. 31 August 2011.