
Back Aragoniet Afrikaans Aragonito AN أراجونيت Arabic Araqonit Azerbaijani Араганіт Byelorussian Араганіт BE-X-OLD Арагонит Bulgarian Aragonit BS Aragonita Catalan Aragonit Czech
| Aragonite | |
|---|---|
 Aragonite from Los Molinillos, Cuenca, Spain, sample width about 4 cm | |
| General | |
| Category | Carbonate minerals |
| Formula (repeating unit) | Ca CO3 |
| IMA symbol | Arg (not to be confused with arginine)[1] |
| Crystal system | Orthorhombic |
| Unit cell | l a = 4.9598(5) Å, b = 7.9641(9) Å, and c = 5.7379(6) Å at 25 °C[2] |
| Identification | |
| Color | Can come in a variety of colors, but commonly red or white |
| Crystal habit | Commonly dendritic or pseudo-hexagonal; can also be acicular, tabular, prismatic, coral-like |
| Twinning | Cyclic on {110}, forms pseudohexagonal aggregates. If polysynthetic, forms fine striations parallel to [110]. |
| Cleavage | Good on [110], Poor on {110}. |
| Fracture | Subconchoidal |
| Tenacity | Very brittle |
| Mohs scale hardness | 3.5–4 |
| Luster | Vitreous, waxy, resinous |
| Streak | White |
| Diaphaneity | Transparent to opaque |
| Specific gravity | 2.94 |
| Optical properties | Biaxial (−) |
| Refractive index | nω = 1.550 nε = 1.650 |
| Birefringence | δ = 0.155 |
| 2V angle | Measured 18–19° |
| Dispersion | Weak |
| Extinction | Parallel |
| Ultraviolet fluorescence | Faint white-blue to blue-violet |
| Solubility | Soluble in acids, and saltwater (but takes longer) |
| Common impurities | Commonly strontium, zirconium, lead |
| Other characteristics | Thermodynamically unstable, Morphs slowly back into calcite |
| References | [3][4] |
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (Ca CO3), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments.
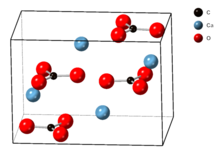
The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal.[5] Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called flos-ferri ("flowers of iron") from their association with the ores at the Carinthian iron mines.[6]
- ^ Warr, L. N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Dickens, B.; Bowen, J. S. (1971). "Refinement of the Crystal Structure of the Aragonite Phase of CaCO(3)". Journal of Research of the National Bureau of Standards Section A. 75A (1): 27–32. doi:10.6028/jres.075A.004. PMC 6715969. PMID 34876711.
- ^ "Aragonite Properties, Occurrence » Geology Science". 26 October 2021.
- ^ Aragonite, Mindat.org
- ^ Bragg, William Lawrence (1924-01-01). "The structure of aragonite". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. 105 (729): 16–39. Bibcode:1924RSPSA.105...16B. doi:10.1098/rspa.1924.0002. ISSN 0950-1207.
- ^ Sinkankas, John (1964). Mineralogy for amateurs. Princeton, N.J.: Van Nostrand. pp. 371–372. ISBN 0442276249.