
Back Aromatiese verbinding Afrikaans مركب أروماتي Arabic Aromatik birləşmələr Azerbaijani Ароматик берләшмәләр Bashkir Aromatikong kompuwesto BCL Араматычныя злучэнні Byelorussian Арэны BE-X-OLD সুগন্ধি যৌগ Bengali/Bangla Aromatski spoj BS Compost aromàtic Catalan
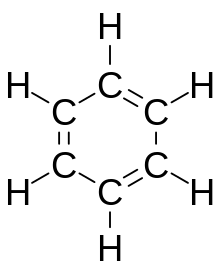
Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."[1] The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
- Typically unreactive
- Often non polar and hydrophobic
- High carbon-hydrogen ratio
- Burn with a strong sooty yellow flame, due to high C:H ratio
- Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions[2]
Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in many reaction types, following both additions and removals, as well as saturation and dearomatization.
- ^ "Aromatic". IUPAC GoldBook. Retrieved 2023-11-06.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1