
Back بنزیل سالیسیلات AZB Salicilat de benzil Catalan Benzylsalicylat Danish Salicylsäurebenzylester German Benzila salikato Esperanto Salicilato de bencilo Spanish بنزیل سالیسیلات Persian Bentsyylisalisylaatti Finnish Salicylate de benzyle French Benzil salicilato Italian
| |

| |
| Names | |
|---|---|
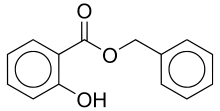
| Preferred IUPAC name
Benzyl 2-hydroxybenzoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.876 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.17 g/cm3 |
| Melting point | 24 to 25 °C (75 to 77 °F; 297 to 298 K) |
| Boiling point | 318 °C (604 °F; 591 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in cosmetics as a fragrance additive or UV light absorber. It appears as an almost colorless liquid with a mild odor described as "very faint, sweet-floral, slightly balsamic" by some, while others smell nothing at all. There is debate whether the odour is caused solely by impurities or a genetic predisposition.[1] It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.[2]
There is some evidence that people may become sensitized to this material[3] and as a result, there is a restriction standard concerning the use of this material in fragrances by the International Fragrance Association.[4]
It is used as a solvent for crystalline synthetic musks and as a component and fixative in floral perfumes such as carnation, jasmine, lilac, and wallflower.[5]
- ^ Steffen Arctander: Perfume and Flavor Chemicals. ISBN 0-931710-37-5
- ^ "Benzyl salicylate". The Good Scents Company.
- ^ Belsito, D; Bickers, D; Bruze, M; Calow, P; Greim, H; Hanifin, JM; Rogers, AE; Saurat, JH; Sipes, IG; Tagami, H (2007). "Toxicologic and Dermatologic Assessments for Three Groups of Fragrance Ingredients: 1) Related Esters and Alcohols of Cinnamic Acid and Cinnamic Alcohol, 2) Ionones, 3) Salicylates" (PDF). Food and Chemical Toxicology. 45 (Supplement 1): S1-23. doi:10.1016/j.fct.2007.09.087. PMID 18035463. Archived from the original (PDF) on 2021-01-12. Retrieved 2012-04-05.
- ^ "Standards Restricted". International Fragrance Association. Archived from the original on 2012-01-04.
- ^ An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN 978-0-9608752-8-3, ISBN 978-1-870228-24-4