
Back جزيء ثنائي الحلقة Arabic Bicyklická molekula Czech Bicyclen German Kondenseerunud tsüklitega molekulid Estonian مولکول دوحلقهای Persian Composé bicyclique French Biciklusos vegyület Hungarian Bicicloalcani Italian 二環式化合物 Japanese 두고리 화합물 Korean
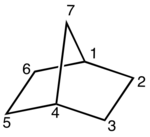

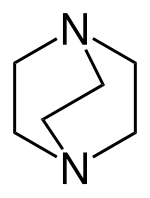
A bicyclic molecule (from bi 'two' and cycle 'ring') is a molecule that features two joined rings.[1] Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO.[2] Moreover, the two rings can both be aliphatic (e.g. decalin and norbornane), or can be aromatic (e.g. naphthalene), or a combination of aliphatic and aromatic (e.g. tetralin).
Three modes of ring junction are possible for a bicyclic compound:[3]
- In spiro compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon.[4] An example of a spirocyclic compound is the photochromic switch spiropyran.
- In fused/condensed[5] bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, i.e. the bridgehead atoms are directly connected (e.g. α-thujene and decalin).
- In bridged bicyclic compounds, the two rings share three or more atoms, separating the two bridgehead atoms by a bridge containing at least one atom. For example, norbornane, also known as bicyclo[2.2.1]heptane, can be viewed as a pair of cyclopentane rings each sharing three of their five carbon atoms. Camphor is a more elaborate example.

- ^ Smith, Michael B. (2011-06-29). Organic Chemistry: An Acid—Base Approach. CRC Press. ISBN 978-1-4200-7921-0.
- ^ "heterocyclic compounds". IUPAC GOLD BOOK. 2014. doi:10.1351/goldbook.H02798.
- ^ Sorrell, Thomas N. (2006). Organic Chemistry. University Science Books. ISBN 978-1-891389-38-2.
- ^ "spiro compounds". IUPAC GOLD BOOK. 2014. doi:10.1351/goldbook.S05881.
- ^ "Aromatic Hydrocarbon - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2021-05-06.