
Back بوتانال Arabic Butanal Azerbaijani بوتیرآلدهید AZB Butanal Catalan Butyraldehyd Czech Butanal German Βουτανάλη Greek Butanalo Esperanto Butanal Spanish بوتیرآلدهید Persian
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Butanal | |
| Other names
Butyraldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.225 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1129 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.107 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Pungent, aldehyde odor |
| Density | 0.8016 g/mL |
| Melting point | −96.86 °C (−142.35 °F; 176.29 K) |
| Boiling point | 74.8 °C (166.6 °F; 347.9 K) |
| Critical point (T, P) | 537 K (264 °C), 4.32 MPa (42.6 atm) |
| 7.6 g/100 mL (20 °C) | |
| Solubility | Miscible with organic solvents |
| log P | 0.88 |
| −46.08·10−6 cm3/mol | |
Refractive index (nD)
|
1.3766 |
| Viscosity | 0.45 cP (20 °C) |
| 2.72 D | |
| Thermochemistry[2] | |
Heat capacity (C)
|
163.7 J·mol−1·K−1 (liquid) 103.4 J·mol−1·K−1 (gas) |
Std molar
entropy (S⦵298) |
246.6 J·mol−1·K−1 (liquid) 343.7 J·mol−1·K−1 (gas) |
Std enthalpy of
formation (ΔfH⦵298) |
−239.2 kJ·mol−1 (liquid) −204.8 kJ·mol−1 (gas) |
Std enthalpy of
combustion (ΔcH⦵298) |
2470.34 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
  [3] [3]
| |
| Danger | |
| H225, H319[3] | |
| P210, P280, P302+P352, P304+P340, P305+P351+P338[3] | |
| NFPA 704 (fire diamond) | |
| Flash point | −7 °C (19 °F; 266 K) |
| 230 °C (446 °F; 503 K) | |
| Explosive limits | 1.9–12.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2490 mg/kg (rat, oral) |
| Safety data sheet (SDS) | Sigma-Aldrich |
| Related compounds | |
Related aldehyde
|
Propionaldehyde Pentanal |
Related compounds
|
Butan-1-ol Butyric acid, isobutyraldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
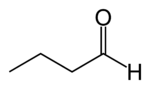
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.
- ^ Merck Index, 11th Edition, 1591.
- ^ CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ a b c Record of Butyraldehyde in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 13 March 2020.
