
Back غاز سي آر Arabic قاز سیآر AZB Dibenzoxazepin German گاز سیآر Persian Dibenzoxazépine French Gas CR Italian CRガス Japanese Dibenzoksazepinas Lithuanian Dibenzoksazepina Polish Dibenzoxazepina Portuguese
| |
| |
| Names | |
|---|---|
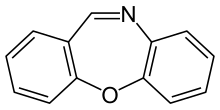
| Preferred IUPAC name
Dibenzo[b,f][1,4]oxazepine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.114.990 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H9NO | |
| Molar mass | 195.221 g·mol−1 |
| Density | 1.160±0.10 g/cm3 |
| Melting point | 73 °C (163 °F; 346 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
CR gas or dibenzoxazepine (chemical name dibenz[b,f][1,4]oxazepine, is an incapacitating agent and a lachrymatory agent. CR was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. A report from the Porton Down laboratories described exposure as "like being thrown blindfolded into a bed of stinging nettles", and it earned the nickname "firegas".[1][2]
In its effects, CR gas is very similar to CS gas (o-chlorobenzylidene malononitrile), but twice as potent, even though there is little structural resemblance between the two. For example, 2 mg of dry CR causes skin redness in 10 min, 5 mg causes burning and erythremia, and 20 mg—strong pain. Water usually amplifies the pain effect of CR on skin. CR aerosols cause irritation at concentrations of 0.2 mcg/L, becoming intolerable at 3 mcg/L. The LD50 of CR through air inhalation 350 mg·min/L.[3]
- ^ Rosenhead, Jonathon (23 Jul 1981). "The technology of riot control". New Scientist.
- ^ Wright, Steve (6 January 1998). "An Appraisal of Technologies of Political Control". The STOA Programme, Directorate General for Research, European Parliament. Retrieved 3 October 2015 – via pitt.edu.
- ^ Aleksandroc V.N., Emelyanov V.I. Poisonous Substances: textbook / G.A. Sokolskiy, ed. Moscow. 1990. — 272 с. ISBN 5-203-00341-6.