
Back سيلينيد الكادميوم Arabic کادمیوم سلنید AZB Seleniur de cadmi Catalan Selenid kademnatý Czech Cadmiumselenid German کادمیم سلنید Persian Kadmiumselenidi Finnish Séléniure de cadmium French कैडमियम सेलेनाइड Hindi Kadmijev selenid Croatian
| |

| |
| Names | |
|---|---|
| IUPAC name
Selanylidenecadmium[2]
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.772 |
| EC Number |
|
| 13656 | |
| MeSH | cadmium+selenide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2570 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CdSe | |
| Molar mass | 191.385 g·mol−1 |
| Appearance | Black, translucent, adamantine crystals |
| Odor | Odorless |
| Density | 5.81 g cm−3[3] |
| Melting point | 1,240 °C (2,260 °F; 1,510 K)[3] |
| Band gap | 1.74 eV, both for hex. and sphalerite[4] |
Refractive index (nD)
|
2.5 |
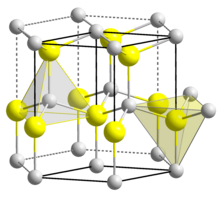
| Structure | |
| Wurtzite | |
| C6v4-P63mc | |
| Hexagonal | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H312, H331, H373, H410 | |
| P261, P273, P280, P301+P310, P311, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1027] TWA 0.005 mg/m3 (as Cd)[5] |
REL (Recommended)
|
Ca[5] |
IDLH (Immediate danger)
|
Ca [9 mg/m3 (as Cd)][5] |
| Related compounds | |
Other anions
|
Cadmium oxide, Cadmium sulfide, Cadmium telluride |
Other cations
|
Zinc selenide, Mercury(II) selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment, but applications are declining because of environmental concerns.[6]
- ^ a b "cadmium selenide (CHEBI:50834)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. IUPAC Names.
- ^ "cadmium selenide – PubChem Public Chemical Database". The PubChem Project. USA: Nation Center for Biotechnology Information. Descriptors Computed from Structure.
- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.54. ISBN 1-4398-5511-0.
- ^ Ninomiya, Susumu; Adachi, Sadao (1995). "Optical properties of cubic and hexagonal Cd Se". Journal of Applied Physics. 78 (7): 4681. Bibcode:1995JAP....78.4681N. doi:10.1063/1.359815.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- ^ Langner, Bernd E. (2000). "Selenium and Selenium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a23_525. ISBN 3527306730.