
Back كانابيديول Arabic کانابیدیول AZB Канабидиол Bulgarian Cannabidiol Catalan U cannabidiol (CBD) Corsican Kanabidiol Czech Cannabidiol German Κανναβιδιόλη Greek Cannabidiol Spanish Kanabidiol Basque
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /kæ.nə.bə.ˈdaɪ.əl/ |
| Trade names | Epidiolex, Epidyolex |
| Other names | CBD, cannabidiolum, (−)-cannabidiol[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618051 |
| License data |
|
| Pregnancy category |
|
| Addiction liability | None [3] |
| Routes of administration | By mouth,[4] sublingual, buccal,[5][6][7]inhalation |
| Drug class | Cannabinoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
|
| Elimination half-life | 18–32 hours[16] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.986 |
| Chemical and physical data | |
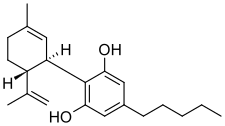
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
| Boiling point | 160–180 °C (320–356 °F) [17][unreliable medical source?] |
| Solubility in water | Insoluble |
| |
| |
| (verify) | |
| Part of a series on |
| Cannabis |
|---|
 |
Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract.[18] Medically, it is an anticonvulsant used to treat multiple forms of epilepsy.[4] It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions.[19][20][21][22] CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.[23]
Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, swallowing it by mouth, and through use of an aerosol spray into the cheek.[5][6] It may be supplied as CBD oil containing only CBD as the active ingredient (excluding THC or terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or prescription liquid solution.[4][21] CBD does not have the same psychoactivity as THC,[24][25] and can modulate the psychoactive effects of THC on the body if both are present.[18][24][26][27] Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.[28]
In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration (FDA) in 2018 for the treatment of two seizure disorders.[4] While the 2018 United States Farm Bill removed hemp and hemp extracts (including CBD) from the Controlled Substances Act, the marketing and sale of CBD formulations for medical use or as an ingredient in dietary supplements or manufactured foods remains illegal under FDA regulation, as of 2024[update].[29][30]
- ^ "cannabidiol (CHEBI:69478)". ebi.ac.uk. Archived from the original on May 12, 2021. Retrieved February 12, 2019.
- ^ "Epidyolex". Therapeutic Goods Administration (TGA). September 29, 2020. Archived from the original on October 30, 2021. Retrieved September 30, 2020.
- ^ "Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements". Federal Register, US Federal Government. September 28, 2018. Retrieved March 10, 2024.
- ^ a b c d e "Epidiolex – cannabidiol solution". DailyMed. August 26, 2020. Archived from the original on February 25, 2021. Retrieved September 11, 2020.
- ^ a b Itin C, Barasch D, Domb AJ, Hoffman A (May 2020). "Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol". International Journal of Pharmaceutics. 581: 119276. doi:10.1016/j.ijpharm.2020.119276. PMID 32243971. S2CID 214785913.
- ^ a b Itin C, Domb AJ, Hoffman A (October 2019). "A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states". Expert Opinion on Drug Delivery. 16 (10): 1031–1035. doi:10.1080/17425247.2019.1653852. PMID 31393180. S2CID 199505274.
- ^ "Sativex (Cannabidiol/Tetrahydrocannabinol) Bayer Label" (PDF). bayer.ca. Archived (PDF) from the original on January 16, 2021. Retrieved June 28, 2018.
- ^ Anvisa (July 24, 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published July 25, 2023). Archived from the original on August 27, 2023. Retrieved August 27, 2023.
- ^ Cite error: The named reference
Home Officewas invoked but never defined (see the help page). - ^ "DEA announces steps necessary to improve access to marijuana research". DEA. August 26, 2019. Retrieved September 7, 2024.
- ^ "7 U.S. Code § 1639o". LII / Legal Information Institute. Retrieved September 7, 2024.
- ^ Laurence E (May 31, 2022). "Your Guide To CBD Legalization By State". Forbes Health. Retrieved September 7, 2024.
- ^ Cite error: The named reference
Epidyolex EPARwas invoked but never defined (see the help page). - ^ Perucca E, Bialer M (June 5, 2020). "Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications". CNS Drugs. 34 (8): 795–800. doi:10.1007/s40263-020-00741-5. PMID 32504461. S2CID 219313952.
- ^ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytotherapy Research (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286. S2CID 21836765. Archived from the original on April 11, 2021. Retrieved May 22, 2020.
- ^ Cite error: The named reference
devinskywas invoked but never defined (see the help page). - ^ "Phytocannabinoid Boiling Points" (PDF). Project CBD. Archived (PDF) from the original on April 8, 2019. Retrieved August 20, 2021.
- ^ a b Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 367 (1607): 3364–3378. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
- ^ Kirkland AE, Fadus MC, Gruber SA, Gray KM, Wilens TE, Squeglia LM (February 2022). "A scoping review of the use of cannabidiol in psychiatric disorders". Psychiatry Research. 308: 114347. doi:10.1016/j.psychres.2021.114347. PMC 8799523. PMID 34952255.
- ^ Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. (December 2019). "Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis". The Lancet. Psychiatry. 6 (12): 995–1010. doi:10.1016/S2215-0366(19)30401-8. PMC 6949116. PMID 31672337.
- ^ a b VanDolah HJ, Bauer BA, Mauck KF (September 2019). "Clinicians' Guide to Cannabidiol and Hemp Oils". Mayo Clinic Proceedings. 94 (9): 1840–1851. doi:10.1016/j.mayocp.2019.01.003. PMID 31447137.
- ^ Prud'homme M, Cata R, Jutras-Aswad D (January 2015). "Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence". Substance Abuse. 9: 33–38. doi:10.4137/SART.S25081. PMC 4444130. PMID 26056464.
- ^ Cite error: The named reference
sbmwas invoked but never defined (see the help page). - ^ a b Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. (July 2017). "Cannabidiol: State of the art and new challenges for therapeutic applications". Pharmacology & Therapeutics. 175: 133–150. doi:10.1016/j.pharmthera.2017.02.041. PMID 28232276.
- ^ Iseger TA, Bossong MG (March 2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophrenia Research. 162 (1–3): 153–161. doi:10.1016/j.schres.2015.01.033. PMID 25667194. S2CID 3745655.
- ^ Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M (January 2018). "Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol". Neuropsychopharmacology. 43 (1): 142–154. doi:10.1038/npp.2017.209. PMC 5719112. PMID 28875990.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. hdl:1874/350973. PMID 26836472. Archived from the original on January 5, 2023. Retrieved November 27, 2022.
- ^ Czégény Z, Nagy G, Babinszki B, Bajtel Á, Sebestyén Z, Kiss T, et al. (April 2021). "CBD, a precursor of THC in e-cigarettes". Scientific Reports. 11 (1): 8951. Bibcode:2021NatSR..11.8951C. doi:10.1038/s41598-021-88389-z. PMC 8076212. PMID 33903673.
- ^ Mead A (June 14, 2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science. 10: 697. doi:10.3389/fpls.2019.00697. PMC 6590107. PMID 31263468.
- ^ "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). #2. How does the 2018 Farm Bill define hemp? What does it mean for FDA-regulated products?". US Food and Drug Administration (FDA). February 6, 2024. Retrieved February 6, 2024.