Back Koolstofdioksied Afrikaans Kohlenstoffdioxid ALS ካርቦን ክልቶኦክሳይድ Amharic Dioxido de carbonio AN ثنائي أكسيد الكربون Arabic কাৰ্বন ডাই অক্সাইড Assamese Dióxidu de carbonu AST Karbon qazı Azerbaijani کربون دیاوکسید AZB Onglėis dvėdegėnis BAT-SMG

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbon dioxide
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1900390 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.271 | ||
| EC Number |
| ||
| E number | E290 (preservatives) | ||
| 989 | |||
| KEGG | |||
| MeSH | Carbon+dioxide | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1013 (gas), 1845 (solid) | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CO2 | |||
| Molar mass | 44.009 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor |
| ||
| Density |
| ||
| Critical point (T, P) | 304.128(15) K[2] (30.978(15) °C), 7.3773(30) MPa[2] (72.808(30) atm) | ||
| 194.6855(30) K (−78.4645(30) °C) at 1 atm (0.101325 MPa) | |||
| 1.45 g/L at 25 °C (77 °F), 100 kPa (0.99 atm) | |||
| Vapor pressure | 5.7292(30) MPa, 56.54(30) atm (20 °C (293.15 K)) | ||
| Acidity (pKa) | Carbonic acid: pKa1 = 3.6 pKa1(apparent) = 6.35 pKa2 = 10.33 | ||
| −20.5·10−6 cm3/mol | |||
| Thermal conductivity | 0.01662 W·m−1·K−1 (300 K (27 °C; 80 °F))[3] | ||
Refractive index (nD)
|
1.00045 | ||
| Viscosity |
| ||
| 0 D | |||
| Structure | |||
| Trigonal | |||
| Linear | |||
| Thermochemistry | |||
Heat capacity (C)
|
37.135 J/(K·mol) | ||
Std molar
entropy (S⦵298) |
214 J·mol−1·K−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−393.5 kJ·mol−1 | ||
| Pharmacology | |||
| V03AN02 (WHO) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
90,000 ppm (162,000 mg/m3) (human, 5 min)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5000 ppm (9000 mg/m3)[5] | ||
REL (Recommended)
|
TWA 5000 ppm (9000 mg/m3), ST 30,000 ppm (54,000 mg/m3)[5] | ||
IDLH (Immediate danger)
|
40,000 ppm (72,000 mg/m3)[5] | ||
| Safety data sheet (SDS) | Sigma-Aldrich | ||
| Related compounds | |||
Other anions
|
|||
Other cations
|
|||
| See Oxocarbon | |||
Related compounds
|
|||
| Supplementary data page | |||
| Carbon dioxide (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
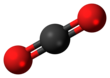
Carbon dioxide is a chemical compound with the chemical formula CO2. It is made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature and at normally-encountered concentrations it is odorless.. As the source of carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater.
It is a trace gas in Earth's atmosphere at 421 parts per million (ppm)[a], or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%.[10][11] Burning fossil fuels is the main cause of these increased CO2 concentrations, which are the primary cause of climate change.[12]
Its concentration in Earth's pre-industrial atmosphere since late in the Precambrian was regulated by organisms and geological features. Plants, algae and cyanobacteria use energy from sunlight to synthesize carbohydrates from carbon dioxide and water in a process called photosynthesis, which produces oxygen as a waste product.[13] In turn, oxygen is consumed and CO2 is released as waste by all aerobic organisms when they metabolize organic compounds to produce energy by respiration.[14] CO2 is released from organic materials when they decay or combust, such as in forest fires. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate (HCO−3), which causes ocean acidification as atmospheric CO2 levels increase.[15]
Carbon dioxide is 53% more dense than dry air, but is long lived and thoroughly mixes in the atmosphere. About half of excess CO2 emissions to the atmosphere are absorbed by land and ocean carbon sinks.[16] These sinks can become saturated and are volatile, as decay and wildfires result in the CO2 being released back into the atmosphere.[17] CO2 is eventually sequestered (stored for the long term) in rocks and organic deposits like coal, petroleum and natural gas.
Nearly all CO2 produced by humans goes into the atmosphere. Less than 1% of CO2 produced annually is put to commercial use, mostly in the fertilizer industry and in the oil and gas industry for enhanced oil recovery. Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses. [18]: 3
- ^ Cite error: The named reference
AirProductsMSDSwas invoked but never defined (see the help page). - ^ a b Span R, Wagner W (1 November 1996). "A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa". Journal of Physical and Chemical Reference Data. 25 (6): 1519. Bibcode:1996JPCRD..25.1509S. doi:10.1063/1.555991.
- ^ Touloukian YS, Liley PE, Saxena SC (1970). "Thermophysical properties of matter - the TPRC data series". Thermal Conductivity - Nonmetallic Liquids and Gases. 3. Data book.
- ^ Schäfer M, Richter M, Span R (2015). "Measurements of the viscosity of carbon dioxide at temperatures from (253.15 to 473.15) K with pressures up to 1.2 MPa". The Journal of Chemical Thermodynamics. 89: 7–15. Bibcode:2015JChTh..89....7S. doi:10.1016/j.jct.2015.04.015. ISSN 0021-9614.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0103". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Carbon dioxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Safety Data Sheet – Carbon Dioxide Gas – version 0.03 11/11" (PDF). AirGas.com. 12 February 2018. Archived (PDF) from the original on 4 August 2018. Retrieved 4 August 2018.
- ^ "Carbon dioxide, refrigerated liquid" (PDF). Praxair. p. 9. Archived from the original (PDF) on 29 July 2018. Retrieved 26 July 2018.
- ^ "CO2 Gas Concentration Defined". CO2 Meter. 18 November 2022. Retrieved 5 September 2023.
- ^ Eggleton T (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763. Retrieved 9 November 2020.
- ^ "Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration". www.noaa.gov. 3 June 2022. Retrieved 14 June 2022.
- ^ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, US
- ^ Kaufman DG, Franz CM (1996). Biosphere 2000: protecting our global environment. Kendall/Hunt Pub. Co. ISBN 978-0-7872-0460-0.
- ^ "Food Factories". www.legacyproject.org. Archived from the original on 12 August 2017. Retrieved 10 October 2011.
- ^ Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. Washington, DC: National Academies Press. 22 April 2010. pp. 23–24. doi:10.17226/12904. ISBN 978-0-309-15359-1. Archived from the original on 5 February 2016. Retrieved 29 February 2016.
- ^ IPCC (2021). "Summary for Policymakers" (PDF). Climate Change 2021: The Physical Science Basis. p. 20. Archived (PDF) from the original on 10 October 2022.
- ^ Myles, Allen (September 2020). "The Oxford Principles for Net Zero Aligned Carbon Offsetting" (PDF). Archived (PDF) from the original on 2 October 2020. Retrieved 10 December 2021.
- ^ "Putting CO2 to Use – Analysis". IEA. 25 September 2019. Retrieved 30 October 2024.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).