
Back Kobalt-60 Afrikaans كوبالت-60 Arabic Кобальт-60 Byelorussian Cobalt 60 Catalan Kobalt-60 Czech Cobalto-60 Spanish کبالت-۶۰ Persian Koboltti-60 Finnish Cobalt 60 French Cobalto-60 Galician
 | |
| General | |
|---|---|
| Symbol | 60Co |
| Names | cobalt-60, 60Co, Co-60 |
| Protons (Z) | 27 |
| Neutrons (N) | 33 |
| Nuclide data | |
| Natural abundance | trace |
| Half-life (t1/2) | 5.27 years[1] |
| Isotope mass | 59.9338222 Da |
| Spin | 5+ |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β (beta decay) | 0.317[2] |
| γ (gamma-rays) | 1.1732,1.3325 |
| Isotopes of cobalt Complete table of nuclides | |
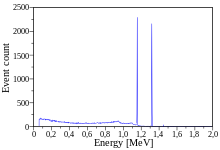
Cobalt-60 (60Co) is a synthetic radioactive isotope of cobalt with a half-life of 5.2714 years.[3][4]: 39 It is produced artificially in nuclear reactors. Deliberate industrial production depends on neutron activation of bulk samples of the monoisotopic and mononuclidic cobalt isotope 59
Co
.[5] Measurable quantities are also produced as a by-product of typical nuclear power plant operation and may be detected externally when leaks occur. In the latter case (in the absence of added cobalt) the incidentally produced 60
Co
is largely the result of multiple stages of neutron activation of iron isotopes in the reactor's steel structures[6] via the creation of its 59
Co
precursor. The simplest case of the latter would result from the activation of 58
Fe
. 60
Co
undergoes beta decay to the stable isotope nickel-60 (60
Ni
). The activated cobalt nucleus emits two gamma rays with energies of 1.17 and 1.33 MeV, hence the overall equation of the nuclear reaction (activation and decay) is: 59
27Co
+ n → 60
27Co
→ 60
28Ni
+ e− + 2 γ
- ^ "Radionuclide Half-Life Measurements". National Institute of Standards and Technology. Archived from the original on 12 August 2016. Retrieved 7 November 2011.
- ^ "Chart of Nucleids". National Nuclear Data Center. Brookhaven National Laboratory. Archived from the original on 22 May 2008. Retrieved 25 October 2018.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Eckerman, K.; Endo, A. (2008). Annex A. Radionuclides of the ICRP-07 collection. ICRP Publication 107. Vol. 38. International Commission on Radiological Protection. pp. 35–96. doi:10.1016/j.icrp.2008.10.002. ISBN 978-0-7020-3475-6. ISSN 0146-6453. LCCN 78647961. PMID 19285593.
{{cite book}}:|journal=ignored (help) - ^ Malkoske, G. R.; Slack, J.; Norton, J. L. (2–5 June 2002). Cobalt-60 production in CANDU power reactors. 40 years of nuclear energy in Canada = 40 anne´es d'e´nergie nucle´aire au Canada (Conference). Vol. 34. Canadian Nuclear Society. pp. 96Megabytes. ISBN 978-0919784697. OCLC 59260021 – via International Atomic Energy Agency. (PDF also located at Canadian Nuclear FAQ)
- ^ US EPA Radiation Protection: Cobalt