
Back تفاعل تكاثف Arabic Atmosferdə kondensasiya nüvəsi Azerbaijani Кондензация (химия) Bulgarian ঘনীভবন বিক্রিয়া Bengali/Bangla Reacció de condensació Catalan کاردانەوەی خەستبوونەوە CKB Kondenzace (chemie) Czech Kondensationsreaktion Danish Kondensationsreaktion German Αντίδραση συμπύκνωσης Greek
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water.[1] If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.[2]
The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation).[3] The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.[4]
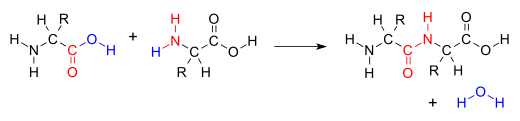
Many variations of condensation reactions exist. Common examples include the aldol condensation and the Knoevenagel condensation, which both form water as a by-product, as well as the Claisen condensation and the Dieckman condensation (intramolecular Claisen condensation), which form alcohols as by-products.[5]
- ^ "25.18 Condensation Reactions". Book: Introductory Chemistry (CK-12). Chemistry Libre Texts. 12 August 2020. Retrieved 9 January 2021.
- ^ "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. 2014. doi:10.1351/goldbook.C01238. Retrieved 7 December 2017.
- ^ Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700. S2CID 105101288.
- ^ Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
