
Back Dializ (geoloji termin) Azerbaijani Дыяліз Byelorussian Диализа (биохимия) Bulgarian Dialyse (Chemie) German Diálisis (bioquímica) Spanish Dialüüs Estonian דיאליזה (כימיה) HE Դիալիզ Armenian Dialisi (fisica) Italian Диализ Kirghiz
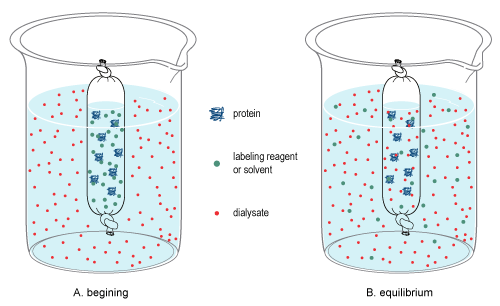
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.[1]
Dialysis is a common laboratory technique that operates on the same principle as medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides.[2] Dialysis is also commonly used for buffer exchange and drug binding studies.
The concept of dialysis was introduced in 1861 by the Scottish chemist Thomas Graham.[3] He used this technique to separate sucrose (small molecule) and gum Arabic solutes (large molecule) in aqueous solution. He called the diffusible solutes crystalloids and those that would not pass the membrane colloids.[4]
From this concept dialysis can be defined as a spontaneous separation process of suspended colloidal particles from dissolved ions or molecules of small dimensions through a semi permeable membrane. Most common dialysis membrane are made of cellulose, modified cellulose or synthetic polymer (cellulose acetate or nitrocellulose).[5]
- ^ Reed, R (2007). Practical Skills in Biomolecular Sciences (3rd ed.). Essex: Pearson Education Limited. p. 379. ISBN 978-0-13-239115-3.
- ^ Berg, JM (2007). Biochemistry (6th ed.). New York: W.H. Freeman and Company. p. 69. ISBN 978-0-7167-8724-2.
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 8 (11th ed.). Cambridge University Press. p. 157.
- ^ Cite error: The named reference
:4was invoked but never defined (see the help page). - ^ Ninfa, A.J.; Ballou, D. P.; Benore, M. (2009). Fundamental Laboratory Approaches for Biochemistry and Biotechnology. John Wiley & Sons. p. 45. ISBN 978-0-470-08766-4.