
Back Diëtieleter Afrikaans ثنائي إيثيل الإيثر Arabic Dietil efiri Azerbaijani دیاتیل اتر AZB Дыэтылавы эфір Byelorussian Диетилов етер Bulgarian Dietil eter BS Èter dietílic Catalan Diethylether Czech Diethylether Danish
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
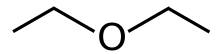
Ethoxyethane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1696894 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.425 |
| EC Number |
|
| 25444 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1155 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O | |
| Molar mass | 74.123 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Dry, Rum-like, sweetish odor[1] |
| Density | 0.7134 g/cm3, liquid |
| Melting point | −116.3 °C (−177.3 °F; 156.8 K) |
| Boiling point | 34.6 °C (94.3 °F; 307.8 K)[4] |
| 6.05 g/(100 mL)[2] | |
| log P | 0.98[3] |
| Vapor pressure | 440 mmHg (58.66 kPa) at 20 °C[1] |
| −55.1·10−6 cm3/mol | |
Refractive index (nD)
|
1.353 (20 °C) |
| Viscosity | 0.224 cP (25 °C) |
| Structure | |
| 1.15 D (gas) | |
| Thermochemistry | |
Heat capacity (C)
|
172.5 J/(mol·K) |
Std molar
entropy (S⦵298) |
253.5 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−271.2 ± 1.9 kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
−2732.1 ± 1.9 kJ/mol |
| Pharmacology | |
| N01AA01 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely flammable, harmful to skin, decomposes to explosive peroxides in air and light[1] |
| GHS labelling: | |
 
| |
| Danger | |
| H224, H302, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P303+P361+P353, P304+P340, P312, P330, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −45 °C (−49 °F; 228 K)[7] |
| 160 °C (320 °F; 433 K)[7] | |
| Explosive limits | 1.9–48.0%[5] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
73,000 ppm (rat, 2 hr) 6500 ppm (mouse, 1.65 hr)[6] |
LCLo (lowest published)
|
106,000 ppm (rabbit) 76,000 ppm (dog)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 400 ppm (1200 mg/m3)[1] |
REL (Recommended)
|
No established REL[1] |
IDLH (Immediate danger)
|
1900 ppm[1] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related ethers
|
|
Related compounds
|
|
| Supplementary data page | |
| Diethyl ether (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethyl ether, or simply ether, is an organic compound with the chemical formula (CH3CH2)2O, sometimes abbreviated as Et2O.[a] It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent. It was formerly used as a general anesthetic.[8]
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0277". National Institute for Occupational Safety and Health (NIOSH).
- ^ Merck Index, 10th ed., Martha Windholz, editor, Merck & Co., Inc, Rahway, NJ, 1983, p. 551
- ^ "Diethyl ether_msds".
- ^ "Diethyl ether". ChemSpider. Retrieved 19 January 2017.
- ^ Carl L. Yaws, Chemical Properties Handbook, McGraw-Hill, New York, 1999, p. 567
- ^ a b "Ethyl ether". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Ethyl Ether MSDS". J.T. Baker. Archived from the original on 2012-03-28. Retrieved 2010-06-24.
- ^ Sakuth, Michael; Mensing, Thomas; Schuler, Joachim; Heitmann, Wilhelm; Strehlke, Günther; Mayer, Dieter (2010). "Ethers, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_023.pub2. ISBN 978-3-527-30385-4.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
