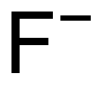Back Fluoried Afrikaans فلوريد Arabic فلورید AZB ফ্লোরাইড Bengali/Bangla Fluorur Catalan Fluoridy Czech Fluoride German ފްލޮރައިޑް DV Fluoruro Spanish Fluoriidid Estonian
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Fluoride[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 14905 | |||
| KEGG | |||
| MeSH | Fluoride | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| F− | |||
| Molar mass | 18.998403163 g·mol−1 | ||
| Conjugate acid | Hydrogen fluoride | ||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
145.58 J/mol K (gaseous)[2] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−333 kJ mol−1 | ||
| Related compounds | |||
Other anions
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fluoride (/ˈflʊəraɪd, ˈflɔːr-/)[3] is an inorganic, monatomic anion of fluorine, with the chemical formula F−
(also written [F]−
), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin.
Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature.
- ^ "Fluorides – PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. Identification.
- ^ Chase, M. W. (1998). "Fluorine anion". NIST. pp. 1–1951. Retrieved 4 July 2012.
- ^ Wells, J.C. (2008). Longman pronunciation dictionary (3rd ed.). Harlow, England: Pearson Education Limited/Longman. p. 313. ISBN 9781405881180.. According to this source, /ˈfluːəraɪd/ is a possible pronunciation in British English.