
Back جينتيوبيوز Arabic جنتیوبیوس AZB Gentiobiosa Catalan Gentiobiose German Gentiobiosa Spanish جنتیوبیوس Persian Gentiobiose French Xentiobiosa Galician ג'נטיביוסה HE Gentiobiosio Italian
| |
| Names | |
|---|---|
| IUPAC name
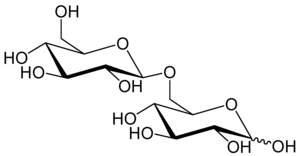
6-O-β-D-glucopyranosyl-D-glucose
| |
| Other names
amygdalose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
| Density | 1.768 g/mL |
| Melting point | 190 to 195 °C (374 to 383 °F; 463 to 468 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Gentiobiose is a disaccharide composed of two units of D-glucose joined with a β(1->6) linkage. It is a white crystalline solid that is soluble in water or hot methanol. Gentiobiose is incorporated into the chemical structure of crocin, the chemical compound that gives saffron its color. It is a product of the caramelization of glucose.[2] During a starch hydrolysis process for glucose syrup, gentiobiose, which has bitterness, is formed as an undesirable product through the acid-catalyzed condensation reaction of two D-glucose molecules.[3] One β-D-glucose unit elongation of the bitter disaccharide reduces its bitterness by a fifth, as determined by human volunteers using the trimer, gentiotriose.[4] Gentiobiose is also produced via enzymatic hydrolysis of glucans, including pustulan[5] and β-1,3-1,6-glucan.[6]
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, p. 4288, ISBN 091191028X
- ^ Sugisawa, Hirqshi; Edo, Hiroshi (1966). "The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose". Journal of Food Science. 31 (4): 561. doi:10.1111/j.1365-2621.1966.tb01905.x.
- ^ Berlin, Henry (1926). "The Occurrence of Gentiobiose in the Products of the Commercial Hydrolysis of Corn Starch2". Journal of the American Chemical Society. 48 (10): 2627–2630. doi:10.1021/ja01421a017.
- ^ Fujimoto, Yoshinori; Hattori, Takeshi; Uno, Shuji; Murata, Takeomi; Usui, Taichi (2009). "Enzymatic synthesis of gentiooligosaccharides by transglycosylation with β-glycosidases from Penicillium multicolor". Carbohydrate Research. 344 (8): 972–978. doi:10.1016/j.carres.2009.03.006. hdl:10297/3621. PMID 19362709.
- ^ Hattori, Takeshi; Kato, Yasuna; Uno, Shuji; Usui, Taichi (2013). "Mode of action of a β-(1→6)-glucanase from Penicillium multicolor". Carbohydrate Research. 366: 6–16. doi:10.1016/j.carres.2012.11.002. PMID 23246473.
- ^ Hirabayashi, Katsuki; Tashiro, Yoshiya; Kondo, Nobuhiro; Hayashi; Sachio (2017). "Production of Gentiobiose from Hydrothermally Treated Aureobasidium pullulans β-1,3-1,6-Glucan". Journal of Applied Glycoscience. 64 (2): 33–37. doi:10.5458/jag.jag.JAG-2017_002. PMC 8056935. PMID 34354494.