Back Glisien Afrikaans جلايسين Arabic Aminosirkə turşusu Azerbaijani قلیسین AZB Гліцын Byelorussian Глицин Bulgarian গ্লাইসিন Bengali/Bangla Glicin BS Glicina Catalan Glycin Czech
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glycine
| |||
| Systematic IUPAC name
Aminoacetic acid[2] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Gly, G | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.248 | ||
| EC Number |
| ||
| E number | E640 (flavour enhancer) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.067 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.1607 g/cm3[3] | ||
| Melting point | 233 °C (451 °F; 506 K) (decomposition) | ||
| 249.9 g/L (25 °C)[4] | |||
| Solubility | soluble in pyridine sparingly soluble in ethanol insoluble in ether | ||
| Acidity (pKa) | 2.34 (carboxyl), 9.6 (amino)[5] | ||
| -40.3·10−6 cm3/mol | |||
| Pharmacology | |||
| B05CX03 (WHO) | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2600 mg/kg (mouse, oral) | ||
| Supplementary data page | |||
| Glycine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
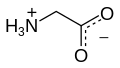
Glycine (symbol Gly or G;[6] /ˈɡlaɪsiːn/ )[7] is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable). Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG).[8] Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group. Glycine is also an inhibitory neurotransmitter[9] – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.[10]
It is the only achiral proteinogenic amino acid.[11] It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.[12]
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 4386
- ^ "Glycine". PubChem.
- ^ Handbook of Chemistry and Physics, CRC Press, 59th edition, 1978.[page needed]
- ^ "Solubilities and densities". Prowl.rockefeller.edu. Archived from the original on September 12, 2017. Retrieved November 13, 2013.
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.[page needed]
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on October 9, 2008. Retrieved March 5, 2018.
- ^ "Glycine | Definition of glycine in English by Oxford Dictionaries". Archived from the original on January 29, 2018.
- ^ Pawlak K, Błażej P, Mackiewicz D, Mackiewicz P (January 2023). "The Influence of the Selection at the Amino Acid Level on Synonymous Codon Usage from the Viewpoint of Alternative Genetic Codes". International Journal of Molecular Sciences. 24 (2): 1185. doi:10.3390/ijms24021185. PMC 9866869. PMID 36674703.
- ^ Zafra F, Aragón C, Giménez C (June 1997). "Molecular biology of glycinergic neurotransmission". Molecular Neurobiology. 14 (3): 117–142. doi:10.1007/BF02740653. PMID 9294860.
- ^ Atchison W (2018). "Toxicology of the Neuromuscular Junction". Comprehensive Toxicology. pp. 259–282. doi:10.1016/B978-0-12-801238-3.99198-0. ISBN 978-0-08-100601-6.
- ^ Matsumoto A, Ozaki H, Tsuchiya S, Asahi T, Lahav M, Kawasaki T, et al. (April 2019). "Achiral amino acid glycine acts as an origin of homochirality in asymmetric autocatalysis". Organic & Biomolecular Chemistry. 17 (17): 4200–4203. doi:10.1039/C9OB00345B. PMID 30932119.
- ^ Alves A, Bassot A, Bulteau AL, Pirola L, Morio B (June 2019). "Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases". Nutrients. 11 (6): 1356. doi:10.3390/nu11061356. PMC 6627940. PMID 31208147.