
Back غوانين Arabic Quanin Azerbaijani قوانین AZB Гуанін Byelorussian Гуанин Bulgarian গুয়ানিন Bengali/Bangla Guanin Breton Guanin BS Guanina Catalan Guanin Czech
| |||
| |||
| Names | |||
|---|---|---|---|
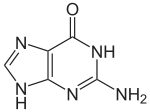
| Preferred IUPAC name
2-Amino-1,9-dihydro-6H-purin-6-one | |||
| Other names
2-amino-6-hydroxypurine,
2-aminohypoxanthine, Guanine | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 147911 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.727 | ||
| EC Number |
| ||
| 431879 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5N5O | |||
| Molar mass | 151.13 g/mol | ||
| Appearance | White amorphous solid. | ||
| Density | 2.200 g/cm3 (calculated) | ||
| Melting point | 360 °C (680 °F; 633 K) decomposes | ||
| Boiling point | Sublimes | ||
| Insoluble. | |||
| Acidity (pKa) | 3.3 (amide), 9.2 (secondary), 12.3 (primary)[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Related compounds
|
Cytosine; Adenine; Thymine; Uracil | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Guanine (/ˈɡwɑːniːn/ ) (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.
With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar.
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.


