
Back Immunoterapia AN علاج مناعي Arabic Имунотерапия Bulgarian Imunoterapija BS Immunoteràpia Catalan Imunoterapie Czech Immuntherapie German Ανοσοθεραπεία Greek Inmunoterapia Spanish Immunoteraapia Estonian
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
| Immunotherapy | |
|---|---|
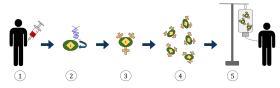 The diagram above represents the process of chimeric antigen receptor T-cell therapy (CAR), this is a method of immunotherapy, which is a growing practice in the treatment of cancer. The final result should be a production of equipped T-cells that can recognize and fight the infected cancer cells in the body.
| |
| MeSH | D007167 |
| OPS-301 code | 8-03 |
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.[1][2][3][4]
Cell-based immunotherapies are effective for some cancers.[5][6] Immune effector cells such as lymphocytes, macrophages, dendritic cells, natural killer cells, and cytotoxic T lymphocytes work together to defend the body against cancer by targeting abnormal antigens expressed on the surface of tumor cells. Vaccine-induced immunity to COVID-19 relies mostly on an immunomodulatory T-cell response.[7]
Therapies such as granulocyte colony-stimulating factor (G-CSF), interferons, imiquimod and cellular membrane fractions from bacteria are licensed for medical use. Others including IL-2, IL-7, IL-12, various chemokines, synthetic cytosine phosphate-guanosine (CpG) oligodeoxynucleotides and glucans are involved in clinical and preclinical studies.
- ^ "Immunotherapy | Memorial Sloan Kettering Cancer Center". mskcc.org. Archived from the original on 2019-10-19. Retrieved 2017-07-27.
- ^ Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ^ Conforti L (February 2012). "The ion channel network in T lymphocytes, a target for immunotherapy". Clinical Immunology. 142 (2): 105–106. doi:10.1016/j.clim.2011.11.009. PMID 22189042.
- ^ Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA (August 2019). "Current Diagnosis and Management of Small-Cell Lung Cancer". Mayo Clinic Proceedings. 94 (8): 1599–1622. doi:10.1016/j.mayocp.2019.01.034. PMID 31378235.
- ^ Riley RS, June CH, Langer R, Mitchell MJ (March 2019). "Delivery technologies for cancer immunotherapy". Nature Reviews. Drug Discovery. 18 (3): 175–196. doi:10.1038/s41573-018-0006-z. PMC 6410566. PMID 30622344.
- ^ Li Y, McBride DW, Tang Y, Doycheva D, Zhang JH, Tang Z (September 2023). "Immunotherapy as a treatment for Stroke: Utilizing regulatory T cells". Brain Hemorrhages. 4 (3): 147–153. doi:10.1016/j.hest.2023.02.003. ISSN 2589-238X.
- ^ Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. (May 2021). "SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees". Science Immunology. 6 (59): eabj1750. doi:10.1126/sciimmunol.abj1750. PMC 9268159. PMID 34035118.
