
Back Ioon Afrikaans أيون Arabic Ion AST İon Azerbaijani یون AZB Iyono BCL Іон Byelorussian Іён BE-X-OLD Йон Bulgarian আয়ন Bengali/Bangla
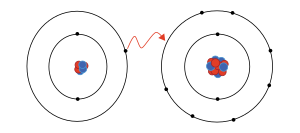
An ion (/ˈaɪ.ɒn, -ən/)[1] is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
A cation is a positively charged ion with fewer electrons than protons[2] (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons.[3] (e.g. Cl- (chloride ion) and OH- (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds.
If only a + or - is present, it indicates a +1 or -1 charge (2+ indicates charge +2, 2- indicates charge -2).[4]
+2 and -2 charge look like this: O22- (negative charge, peroxide) He2+ (positive charge, alpha particle).[4]
Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a positive ion.[5] Ions are also created by chemical interactions, such as the dissolution of a salt in liquids, or by other means, such as passing a direct current through a conducting solution, dissolving an anode via ionization.
- ^ "ion". CollinsDictionary.com. HarperCollins. "Collins English Dictionary | Always Free Online". Archived from the original on 2013-12-24. Retrieved 2013-12-21.
{{cite web}}: CS1 maint: bot: original URL status unknown (link). - ^ "cation". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 2021-10-06. "Definition of CATION". Archived from the original on 2021-10-06. Retrieved 2021-10-06.
{{cite web}}: CS1 maint: bot: original URL status unknown (link). - ^ "anion". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 2021-10-06. "Definition of ANION". Archived from the original on 2021-10-06. Retrieved 2021-10-06.
{{cite web}}: CS1 maint: bot: original URL status unknown (link). - ^ a b "What Is an Ion? Definition and Examples". ThoughtCo. Archived from the original on 2024-08-26. Retrieved 2024-08-26.
- ^ Knoll, Glenn F. (1999). Radiation Detection and Measurement (3rd ed.). New York: Wiley. ISBN 978-0-471-07338-3.