
Back هوموکاپزاسین AZB Isethionsäure German هوموکاپزاسین Persian Acide iséthionique French आइसेथायोनिक अम्ल Hindi Acido isetionico Italian イセチオン酸 Japanese 2-hydroxyethaansulfonzuur Dutch Isetionska kiselina Serbo-Croatian Isetionska kiselina Serbian
| |
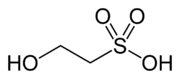
 Isethionic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxyethane-1-sulfonic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.169 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g/mol |
| Density | 1.63 g/cm3 |
| Acidity (pKa) | 1.39 (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isethionic acid is an organosulfur compound containing an alkylsulfonic acid located beta to a hydroxy group. Its discovery is generally attributed to Heinrich Gustav Magnus, who prepared it by the action of solid sulfur trioxide on ethanol in 1833.[1] It is a white water-soluble solid used in the manufacture of certain surfactants and in the industrial production of taurine. It is most commonly available in the form of its sodium salt (sodium isethionate).
- ^ Magnus, G. (1833). "Ueber die Weinschwefelsäure, ihren Einfluss auf die Aetherbildung, und über zwei neue Säuren ähnlicher Zusammensetzung". Annalen der Physik und Chemie. 103 (2): 367–388. Bibcode:1833AnP...103..367M. doi:10.1002/andp.18331030213. ISSN 0003-3804.