
Back لاكتوز Arabic Lactosa AST Laktoza Azerbaijani لاکتوز AZB Лактоза Bashkir Лактоза Byelorussian Ляктоза BE-X-OLD Лактоза Bulgarian ল্যাকটোজ Bengali/Bangla Laktoza BS
| |
| Names | |
|---|---|
| IUPAC name
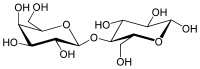
β-D-Galactopyranosyl-(1→4)-D-glucose
| |
| Systematic IUPAC name
(2R,3R,4S,5R,6S)-2-(Hydroxymethyl)-6-{[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxane-3,4,5-triol | |
| Other names
Milk sugar
Lactobiose 4-O-β-D-Galactopyranosyl-D-glucose | |
| Identifiers | |
3D model (JSmol)
|
|
| 90841 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.509 |
| EC Number |
|
| 342369 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Appearance | White solid |
| Density | 1.525 g/cm3 |
| Melting point | 252 °C (anhydrous)[1] 202 °C (monohydrate)[1] |
| 195 g/L[2][3] | |
Chiral rotation ([α]D)
|
+55.4° (anhydrous) +52.3° (monohydrate) |
| Thermochemistry | |
Std enthalpy of
combustion (ΔcH⦵298) |
5652 kJ/mol, 1351 kcal/mol, 16.5 kJ/g, 3.94 kcal/g |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 357.8 °C (676.0 °F; 631.0 K)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lactose, or milk sugar, is a disaccharide composed of galactose and glucose and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from lact (gen. lactis), the Latin word for milk, plus the suffix -ose used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry.[5]
- ^ a b Peter M. Collins (2006). Dictionary of Carbohydrates (2nd ed.). Boca Raton: Chapman & Hall/CRC. p. 677. ISBN 978-0-8493-3829-8.
- ^ "D-Lactose".
- ^ The solubility of lactose in water is 189.049 g at 25 °C, 251.484 g at 40 °C and 372.149 g at 60 °C per kg solution. Its solubility in ethanol is 0.111 g at 40 °C and 0.270 g at 60 °C per kg solution.Machado, José J. B.; Coutinho, João A.; Macedo, Eugénia A. (2001), "Solid–liquid equilibrium of α-lactose in ethanol/water" (PDF), Fluid Phase Equilibria, 173 (1): 121–34, doi:10.1016/S0378-3812(00)00388-5. ds
- ^ Sigma Aldrich
- ^ Gerrit M. Westhoff; Ben F.M. Kuster; Michiel C. Heslinga; Hendrik Pluim; Marinus Verhage (2014). "Lactose and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. pp. 1–9. doi:10.1002/14356007.a15_107.pub2. ISBN 978-3-527-30673-2.
