
Back Vloeibare aardgas Afrikaans غاز طبيعي مسال Arabic Звадкаваны прыродны газ Byelorussian Звадкаваны прыродны газ BE-X-OLD তরলীকৃত প্রাকৃতিক গ্যাস Bengali/Bangla Gas natural liquat Catalan Zkapalněný zemní plyn Czech Liquefied natural gas Danish Flüssigerdgas German Υγροποιημένο φυσικό αέριο Greek


Liquefied natural gas (LNG) is natural gas (predominantly methane, CH4, with some mixture of ethane, C2H6) that has been cooled down to liquid form for ease and safety of non-pressurized storage or transport. It takes up about 1/600th the volume of natural gas in the gaseous state at standard conditions for temperature and pressure.
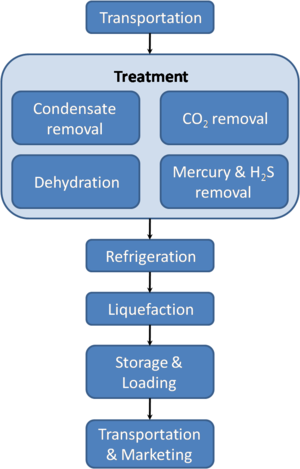
LNG is odorless, colorless, non-toxic and non-corrosive. Hazards include flammability after vaporization into a gaseous state, freezing and asphyxia. The liquefaction process involves removal of certain components, such as dust, acid gases, helium, water, and heavy hydrocarbons, which could cause difficulty downstream. The natural gas is then condensed into a liquid at close to atmospheric pressure by cooling it to approximately −162 °C (−260 °F); maximum transport pressure is set at around 25 kPa (4 psi) (gauge pressure), which is about 1.25 times atmospheric pressure at sea level.
The gas extracted from underground hydrocarbon deposits contains a varying mix of hydrocarbon components, which usually includes mostly methane (CH4), along with ethane (C2H6), propane (C3H8) and butane (C4H10). Other gases also occur in natural gas, notably CO2. These gases have wide-ranging boiling points and also different heating values, allowing different routes to commercialization and also different uses. The "acidic" elements such as hydrogen sulphide (H2S) and carbon dioxide (CO2), together with oil, mud, water, and mercury, are removed from the gas to deliver a clean sweetened stream of gas. Failure to remove much or all of such acidic molecules, mercury, and other impurities could result in damage to the equipment. Corrosion of steel pipes and amalgamization of mercury to aluminum within cryogenic heat exchangers could cause expensive damage.
The gas stream is typically separated into the liquefied petroleum fractions (butane and propane), which can be stored in liquid form at relatively low pressure, and the lighter ethane and methane fractions. These lighter fractions of methane and ethane are then liquefied to make up the bulk of LNG that is shipped.
Natural gas was considered during the 20th century to be economically unimportant wherever gas-producing oil or gas fields were distant from gas pipelines or located in offshore locations where pipelines were not viable. In the past this usually meant that natural gas produced was typically flared, especially since unlike oil, no viable method for natural gas storage or transport existed other than compressed gas pipelines to end users of the same gas. This meant that natural gas markets were historically entirely local, and any production had to be consumed within the local or regional network.
Developments of production processes, cryogenic storage, and transportation effectively created the tools required to commercialize natural gas into a global market which now competes with other fuels. Furthermore, the development of LNG storage also introduced a reliability in networks which was previously thought impossible. Given that storage of other fuels is relatively easily secured using simple tanks, a supply for several months could be kept in storage. With the advent of large-scale cryogenic storage, it became possible to create long term gas storage reserves. These reserves of liquefied gas could be deployed at a moment's notice through regasification processes, and today are the main means for networks to handle local peak shaving requirements.[1]
- ^ Ulvestad, Marte; Overland, Indra (2012). "Natural gas and CO2 price variation: Impact on the relative cost-efficiency of LNG and pipelines". International Journal of Environmental Studies. 69 (3): 407–426. Bibcode:2012IJEnS..69..407U. doi:10.1080/00207233.2012.677581. PMC 3962073. PMID 24683269.