
Back مسكالين Arabic ميسكالين ARZ مسکالین AZB Мескалін Byelorussian Мэскалін BE-X-OLD Мескалин Bulgarian Meskalin Breton Mescalina Catalan Meskalin Czech Meskalin Danish
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Mescalin; Mezcalin; Mezcaline; 3,4,5-Trimethoxyphenethylamine; 3,4,5-TMPEA; TMPEA |
| AHFS/Drugs.com | mescaline |
| Routes of administration | Oral, smoking, insufflation, intravenous[1][2] |
| Drug class | Serotonin receptor agonist; Serotonergic psychedelic; Hallucinogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Oxidative deamination, N-acetylation, O-demethylation, conjugation, other pathways[4][5] |
| Metabolites | • 3,4,5-Trimethoxyphenyl-acetaldehyde[4][1] • 3,4,5-Trimethoxyphenylacetic acid[1] • 3,4,5-Trimethoxyphenylethanol[5] • Others[4][5][2] |
| Onset of action | Oral: 0.5–3 hours[6][1][2] |
| Elimination half-life | 3.6 hours[6][7] |
| Duration of action | ≥10–12 hours[6][1][2] |
| Excretion | Urine (28–81% unchanged, 13–26% as TMPA)[1][4][5][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.174 |
| Chemical and physical data | |
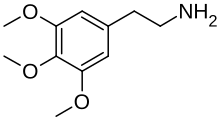
| Formula | C11H17NO3 |
| Molar mass | 211.261 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.067 g/cm3 |
| Melting point | 35 to 36 °C (95 to 97 °F) |
| Boiling point | 180 °C (356 °F) at 12 mmHg |
| Solubility in water | moderately soluble in water mg/mL (20 °C) |
| |
| |
| (verify) | |
Mescaline, also known as mescalin or mezcalin,[8] as well as 3,4,5-trimethoxyphenethylamine, is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class, known for its hallucinogenic effects comparable to those of LSD and psilocybin.[4][1][6][5] It binds to and activates certain serotonin receptors in the brain, producing hallucinogenic effects.[1][6]
- ^ a b c d e f g h Vamvakopoulou IA, Narine KA, Campbell I, Dyck JR, Nutt DJ (January 2023). "Mescaline: The forgotten psychedelic". Neuropharmacology. 222: 109294. doi:10.1016/j.neuropharm.2022.109294. PMID 36252614.
- ^ a b c d e Cite error: The named reference
Dinis-OliveiraPereiradaSilva2019was invoked but never defined (see the help page). - ^ Anvisa (24 July 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 25 July 2023). Archived from the original on 27 August 2023. Retrieved 27 August 2023.
- ^ a b c d e Cassels BK, Sáez-Briones P (October 2018). "Dark Classics in Chemical Neuroscience: Mescaline". ACS Chem Neurosci. 9 (10): 2448–2458. doi:10.1021/acschemneuro.8b00215. PMID 29847089.
- ^ a b c d e Doesburg-van Kleffens M, Zimmermann-Klemd AM, Gründemann C (December 2023). "An Overview on the Hallucinogenic Peyote and Its Alkaloid Mescaline: The Importance of Context, Ceremony and Culture". Molecules. 28 (24): 7942. doi:10.3390/molecules28247942. PMC 10746114. PMID 38138432.
- ^ a b c d e Holze F, Singh N, Liechti ME, D'Souza DC (May 2024). "Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile". Biol Psychiatry Cogn Neurosci Neuroimaging. 9 (5): 472–489. doi:10.1016/j.bpsc.2024.01.007. PMID 38301886.
- ^ Cite error: The named reference
LeyHolzeArikci2023was invoked but never defined (see the help page). - ^ "Mescaline". PubChem. U.S. National Library of Medicine. Retrieved 22 October 2024.