Back ميثيل فينيدات Arabic Metilfenidato AST میتلیففنیدات AZB Метилфенидат Bulgarian Metilfenidat Catalan Methylfenidát Czech Methylffenidad Welsh Methylphenidat Danish Methylphenidat German Μεθυλφαινιδάτη Greek
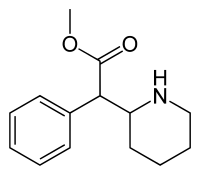
Methylphenidate, sold under the brand names Ritalin (/ˈrɪtəlɪn/ RIT-ə-lin) and Concerta (/kənˈsɜːrtə/ kən-SUR-tə) among others, is a central nervous system (CNS) stimulant used medically to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, narcolepsy. It is a first-line treatment for ADHD (e.g. in the UK[16]); it may be taken by mouth or applied to the skin, and different formulations have varying durations of effect.[4] For ADHD, the effectiveness of methylphenidate is comparable to atomoxetine[17][18][19][20] but modestly lower than amphetamines,[21][22][23][24] alleviating the executive functioning deficits of sustained attention, inhibition, working memory, reaction time[25] and emotional self-regulation.[26][27]
Common adverse reactions of methylphenidate include euphoria, dilated pupils, tachycardia, palpitations, headache, insomnia, anxiety, hyperhidrosis, weight loss, decreased appetite, dry mouth, nausea, and abdominal pain.[10] Withdrawal symptoms may include chills, depression, drowsiness, dysphoria, exhaustion, headache, irritability, lethargy, nightmares, restlessness, suicidal thoughts, and weakness.[4]
Methylphenidate is believed to work by blocking the reuptake of dopamine and norepinephrine by neurons.[28][29] It is a central nervous system (CNS) stimulant of the phenethylamine and piperidine classes. It is available as a generic medication.[30] In 2022, it was the 32nd most commonly prescribed medication in the United States, with more than 17 million prescriptions.[31][32]
- ^ Concerta, "Consumer Medicine Information". New Zealand Medicines and Medical Devices Safety Authority. Retrieved 17 September 2024.
{{cite web}}: Check|url=value (help) - ^ Cite error: The named reference
Hodgkins_2012was invoked but never defined (see the help page). - ^ Stahl SM (April 2024). "Methylphenidate (D,L)". Prescriber's Guide: Stahl's Essential Psychopharmacology (8th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 503–510. ISBN 9781108228749.
- ^ a b c "Methylphenidate Hydrochloride Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 19 December 2018. Retrieved 19 December 2018.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ "Ritalin Product information". Health Canada. 25 April 2012. Archived from the original on 11 June 2022. Retrieved 11 June 2022.
- ^ "Controlled Drugs and Substances Act". Justice Laws Website. 31 March 2022. Archived from the original on 21 August 2021. Retrieved 11 June 2022.
- ^ "Mental health". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ a b "Ritalin- methylphenidate hydrochloride tablet". DailyMed. 26 June 2021. Archived from the original on 20 March 2017. Retrieved 26 March 2022.
- ^ "Ritalin LA- methylphenidate hydrochloride capsule, extended release". DailyMed. 26 June 2021. Archived from the original on 26 March 2017. Retrieved 26 March 2022.
- ^ "Concerta- methylphenidate hydrochloride tablet, extended release". DailyMed. 1 July 2021. Archived from the original on 26 March 2017. Retrieved 26 March 2022.
- ^ "Daytrana- methylphenidate patch". DailyMed. 15 June 2021. Archived from the original on 19 March 2022. Retrieved 26 March 2022.
- ^ Cite error: The named reference
pmid10628897was invoked but never defined (see the help page). - ^ a b "Methylphenidate". Pubchem. Archived from the original on 6 January 2014. Retrieved 4 September 2017.
- ^ "Attention deficit hyperactivity disorder (ADHD): Treatment". National Health Service (NHS). 24 December 2021. Retrieved 18 October 2022.
- ^ Bushe C, Day K, Reed V, Karlsdotter K, Berggren L, Pitcher A, et al. (May 2016). "A network meta-analysis of atomoxetine and osmotic release oral system methylphenidate in the treatment of attention-deficit/hyperactivity disorder in adult patients". Journal of Psychopharmacology. 30 (5): 444–458. doi:10.1177/0269881116636105. PMID 27005307. S2CID 104938.
- ^ Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW (November 2011). "Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis". Journal of Attention Disorders. 15 (8): 674–683. doi:10.1177/1087054710379737. PMID 20837981. S2CID 43503227.
- ^ Hanwella R, Senanayake M, de Silva V (November 2011). "Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis". BMC Psychiatry. 11 (1): 176. doi:10.1186/1471-244X-11-176. PMC 3229459. PMID 22074258.
- ^ Rezaei G, Hosseini SA, Akbari Sari A, Olyaeemanesh A, Lotfi MH, Yassini M, et al. (10 February 2016). "Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review and meta-analysis". Medical Journal of the Islamic Republic of Iran. 30: 325. PMC 4898838. PMID 27390695.
- ^ Stuhec M, Lukić P, Locatelli I (February 2019). "Efficacy, Acceptability, and Tolerability of Lisdexamfetamine, Mixed Amphetamine Salts, Methylphenidate, and Modafinil in the Treatment of Attention-Deficit Hyperactivity Disorder in Adults: A Systematic Review and Meta-analysis". The Annals of Pharmacotherapy. 53 (2): 121–133. doi:10.1177/1060028018795703. PMID 30117329. S2CID 52019992.
- ^ Faraone SV, Pliszka SR, Olvera RL, Skolnik R, Biederman J (June 2001). "Efficacy of Adderall and methylphenidate in attention deficit hyperactivity disorder: a reanalysis using drug-placebo and drug-drug response curve methodology". Journal of Child and Adolescent Psychopharmacology. 11 (2): 171–180. doi:10.1089/104454601750284081. PMID 11436957. ProQuest 204600452.
- ^ Faraone SV, Biederman J, Roe C (October 2002). "Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: a meta-analysis". Journal of Clinical Psychopharmacology. 22 (5): 468–473. doi:10.1097/00004714-200210000-00005. PMID 12352269. S2CID 19726926.
- ^ Faraone SV, Buitelaar J (April 2010). "Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis". European Child & Adolescent Psychiatry. 19 (4): 353–364. doi:10.1007/s00787-009-0054-3. PMID 19763664. S2CID 9447892.
- ^ Isfandnia F, El Masri S, Radua J, Rubia K (July 2024). "The effects of chronic administration of stimulant and non-stimulant medications on executive functions in ADHD: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 162: 105703. doi:10.1016/j.neubiorev.2024.105703. PMID 38718988.
- ^ Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. (September 2021). "The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder". Neuroscience and Biobehavioral Reviews. 128: 789–818. doi:10.1016/j.neubiorev.2021.01.022. PMC 8328933. PMID 33549739.
- ^ Kamradt JM, Ullsperger JM, Nikolas MA (2014). "Executive function assessment and adult attention-deficit/Hyperactivity disorder: Tasks versus ratings on the Barkley Deficits in Executive Functioning Scale". Psychological Assessment. 26 (4): 1095–1105. doi:10.1037/pas0000006. PMID 24885846.
- ^ Arnsten AF, Li BM (June 2005). "Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions". Biological Psychiatry. 57 (11): 1377–1384. doi:10.1016/j.biopsych.2004.08.019. PMID 15950011. S2CID 22992765.
- ^ Stahl SM (11 April 2013). Stahl's Essential Psychopharmacology: Neuroscientific basis and practical applications (4th ed.). Cambridge University Press. ISBN 978-1-107-68646-5.
- ^ "Methylphenidate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 3 February 2019. Retrieved 2 February 2019.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Methylphenidate Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.