
Back ميفيبريستون Arabic الميفيبريستون AZB Mifepriston Czech Miffepriston Welsh Mifepriston German Mifepristona Spanish میفپریستون Persian Mifepristoni Finnish Mifépristone French Mifepriston Croatian
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɪfəˈprɪˌstoʊn/[1] |
| Trade names | Mifegyne, Mifeprex, Korlym, others |
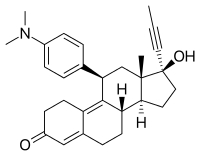
| Other names | RU-486; RU-38486; ZK-98296; 11β-[p-(Dimethylamino)phenyl]-17α-(1-propynyl)estra-4,9-dien-17β-ol-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600042 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antiprogestogen; Antiglucocorticoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 69% |
| Protein binding | 98% |
| Metabolism | Liver |
| Excretion | Feces: 83% urine: 9% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.127.911 |
| Chemical and physical data | |
| Formula | C29H35NO2 |
| Molar mass | 429.604 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.189 g/cm3 |
| Melting point | 194 °C (381 °F) |
| Boiling point | 629 °C (1,164 °F) |
| |
| |
| (verify) | |
Mifepristone, also known by its developmental code name RU-486, is a medication typically used in combination with misoprostol to bring about a medical abortion during pregnancy and manage early miscarriage.[8] This combination is 97% effective during the first 63 days (9 weeks) of pregnancy.[9] It is also effective in the second trimester of pregnancy.[10][11] It is taken by mouth.[8]
The more common adverse effects include abdominal pain, feeling tired, and vaginal bleeding.[8] Serious side effects may include heavy vaginal bleeding, bacterial infection, and birth defects if the pregnancy does not end.[8] If used, appropriate follow-up care needs to be available.[8][12] Mifepristone is an antiprogestogen.[8] It works by blocking the effects of progesterone, making both the cervix and uterine vessels dilate and causing uterine contraction.[8]
Mifepristone was developed in 1980 and came into use in France in 1987.[13] It became available in the United States in 2000.[14][10] It is on the World Health Organization's List of Essential Medicines.[15] Mifepristone was approved in Canada in January 2017.[16][17]
- ^ "mifepristone". Mifepristone Definition & Meaning - Merriam-Webster. Merriam-Webster.com Dictionary. Merriam-Webster. Archived from the original on 3 March 2023. Retrieved 3 March 2023.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Cite error: The named reference
Mifepristone Linepharma PI 2015was invoked but never defined (see the help page). - ^ "Mifegymiso Product information". Health Canada. 10 January 2017. Retrieved 5 September 2024.
- ^ "Mifiso Product information". Health Canada. 16 December 2022. Retrieved 5 September 2024.
- ^ Cite error: The named reference
Korlym FDA labelwas invoked but never defined (see the help page). - ^ "Mifepristone tablet". DailyMed. 30 March 2023. Archived from the original on 10 April 2023. Retrieved 24 April 2023.
- ^ a b c d e f g "Mifepristone". American Society of Health System Pharmacists. Archived from the original on 22 December 2015. Retrieved 25 February 2023 – via Drugs.com.
- ^ Chen MJ, Creinin MD (July 2015). "Mifepristone With Buccal Misoprostol for Medical Abortion: A Systematic Review". Obstetrics and Gynecology. 126 (1): 12–21. doi:10.1097/AOG.0000000000000897. PMID 26241251. S2CID 20800109. Archived from the original on 26 July 2020. Retrieved 27 July 2019 – via eScholarship.
- ^ a b Goldman MB, Troisi R, Rexrode KM, eds. (2012). Women and Health (2nd ed.). Oxford: Academic Press. p. 236. ISBN 978-0-12-384979-3. Archived from the original on 8 September 2017. Retrieved 5 September 2017 – via Google Books.
- ^ Wildschut H, Both MI, Medema S, Thomee E, Wildhagen MF, Kapp N (January 2011). "Medical methods for mid-trimester termination of pregnancy". The Cochrane Database of Systematic Reviews. 2011 (1): CD005216. doi:10.1002/14651858.CD005216.pub2. PMC 8557267. PMID 21249669.
- ^ "Mifepristone Use During Pregnancy". Drugs.com. 20 January 2023. Archived from the original on 23 April 2023. Retrieved 22 April 2023.
- ^ Corey EJ, Czakó B, Kürti L (2012). "Mifepristone". Molecules and Medicine. John Wiley & Sons. ISBN 978-1-118-36173-3. Archived from the original on 8 September 2017 – via Google Books.
- ^ "Drug Approval Package: Mifeprex (Mifepristone) NDA #20687". U.S. Food and Drug Administration (FDA). 18 June 2001. Archived from the original on 21 April 2023. Retrieved 24 April 2023.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Mifepristone Product information". Health Canada. 22 October 2009. Archived from the original on 24 April 2023. Retrieved 24 April 2023.
- ^ Kingston A (5 February 2017). "How the arrival of the abortion pill reveals a double standard". Maclean's. Archived from the original on 21 February 2017. Retrieved 21 February 2017.