
Back ميريكس Arabic میرکس AZB Mirex Catalan Mirex Czech Mirex German Mirex Spanish Mirex Basque میرکس Persian Mireksi Finnish Mirex French
| |

| |
| Names | |
|---|---|
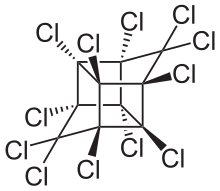
| Preferred IUPAC name
Dodecachlorooctahydro-1H-1,3,4-(epimethanetriyl)cyclobuta[cd]pentalene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.452 |
| EC Number |
|
| KEGG | |
| MeSH | D008917 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10Cl12 | |
| Molar mass | 545.55 g/mol |
| Melting point | 485 °C (905 °F; 758 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mirex is an organochloride that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of cyclopentadiene. It was popularized to control fire ants but by virtue of its chemical robustness and lipophilicity it was recognized as a bioaccumulative pollutant. The spread of the red imported fire ant was encouraged by the use of mirex, which also kills native ants that are highly competitive with the fire ants. The United States Environmental Protection Agency prohibited its use in 1976.[1] It is prohibited by the Stockholm Convention on Persistent Organic Pollutants.
- ^ Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Wienheim, 2002. doi:10.1002/14356007.a14_263