
Back تناظر جزيئي Arabic Simetria molecular Catalan Molekülsymmetrie German Simetría molecular Spanish تقارن مولکولی Persian Symétrie moléculaire French Molekularna simetrija Croatian Simmetria molecolare Italian 分子対称性 Japanese 분자 대칭 Korean
This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Layout of images and tables: need uniform size and formatting, positioning based on how relevant to adjacent content. (July 2023) |
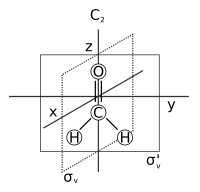
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry.[1][2][3][4][5] Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materials.
There are many techniques for determining the symmetry of a given molecule, including X-ray crystallography and various forms of spectroscopy. Spectroscopic notation is based on symmetry considerations.
- ^ Quantum Chemistry, 3rd ed. John P. Lowe, Kirk Peterson ISBN 0-12-457551-X
- ^ Physical Chemistry: A Molecular Approach by Donald A. McQuarrie, John D. Simon ISBN 0-935702-99-7
- ^ The chemical bond, 2nd ed. J.N. Murrell, S.F.A. Kettle, J.M. Tedder ISBN 0-471-90760-X
- ^ Physical Chemistry, 8th ed. P.W. Atkins and J. de Paula, W.H. Freeman, 2006 ISBN 0-7167-8759-8, chap.12
- ^ G. L. Miessler and D. A. Tarr Inorganic Chemistry, 2nd ed. Pearson, Prentice Hall, 1998 ISBN 0-13-841891-8, chap.4.