Back بروتين مياليني قاعدي Arabic Proteïna bàsica de mielina Catalan MBP Welsh Basisches Myelinprotein German پروتئین اصلی میلین Persian Proteina basica della mielina Italian Basisk myelinprotein NN Основной белок миелина Russian Основний мієліновий білок Ukrainian 髓鞘鹼性蛋白 Chinese
| Myelin_MBP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Myelin_MBP | ||||||||
| Pfam | PF01669 | ||||||||
| InterPro | IPR000548 | ||||||||
| PROSITE | PDOC00492 | ||||||||
| SCOP2 | 1bx2 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 274 | ||||||||
| OPM protein | 2lug | ||||||||
| |||||||||
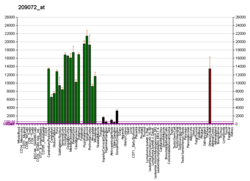
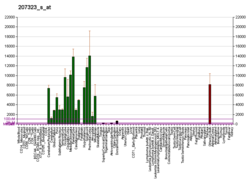
Myelin basic protein (MBP) is a protein believed to be important in the process of myelination of nerves in the nervous system. The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the velocity of axonal impulse conduction.[5] MBP maintains the correct structure of myelin, interacting with the lipids in the myelin membrane.[6][7]
MBP was initially sequenced in 1971 after isolation from bovine myelin membranes.[8] MBP knockout mice called shiverer mice were subsequently developed and characterized in the early 1980s. Shiverer mice exhibit decreased amounts of CNS myelination and a progressive disorder characterized by tremors, seizures, and early death. The human gene for MBP is on chromosome 18;[9] the protein localizes to the CNS and to various cells of the hematopoietic lineage.
The pool of MBP in the central nervous system is very diverse, with several splice variants being expressed and a large number of post-translational modifications on the protein, which include phosphorylation, methylation, deamidation, and citrullination. These forms differ by the presence or the absence of short (10 to 20 residues) peptides in various internal locations in the sequence. In general, the major form of MBP is a protein of about 18.5 Kd (170 residues).
In melanocytic cell types, MBP gene expression may be regulated by MITF.[10]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000197971 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000041607 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Sakamoto Y, Kitamura K, Yoshimura K, Nishijima T, Uyemura K (March 1987). "Complete amino acid sequence of PO protein in bovine peripheral nerve myelin". The Journal of Biological Chemistry. 262 (9): 4208–4214. doi:10.1016/S0021-9258(18)61334-1. PMID 2435734.
- ^ Deber CM, Reynolds SJ (April 1991). "Central nervous system myelin: structure, function, and pathology". Clinical Biochemistry. 24 (2): 113–134. doi:10.1016/0009-9120(91)90421-a. PMC 7130177. PMID 1710177.
- ^ Inouye H, Kirschner DA (January 1991). "Folding and function of the myelin proteins from primary sequence data". Journal of Neuroscience Research. 28 (1): 1–17. doi:10.1002/jnr.490280102. PMID 1710279. S2CID 8598890.
- ^ Cite error: The named reference
pmid5096093was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid2414074was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid19067971was invoked but never defined (see the help page).