Back نترو الميثان Arabic نیترومتان AZB Нитрометан Bulgarian Nitrometà Catalan Nitromethan Czech Nitromethan German Νιτρομεθάνιο Greek Nitrometano Esperanto Nitrometano Spanish نیترومتان Persian
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitromethane
| |||
| Preferred IUPAC name
Nitromethane[1] | |||
| Other names
Nitrocarbol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.797 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3NO2 | |||
| Molar mass | 61.04 g/mol | ||
| Appearance | colorless, oily liquid[2] | ||
| Odor | Light, fruity[2] | ||
| Density | 1.1371 g/cm3 (20 °C)[3] | ||
| Melting point | −28.7 °C (−19.7 °F; 244.5 K)[3] | ||
| Boiling point | 101.2 °C (214.2 °F; 374.3 K)[3] | ||
| Critical point (T, P) | 588 K, 6.0 MPa[4] | ||
| ca. 10 g/100 mL | |||
| Solubility | miscible in diethyl ether, acetone, ethanol, methanol[3] | ||
| Vapor pressure | 28 mmHg (20 °C)[2] | ||
| Acidity (pKa) | |||
| -21.0·10−6 cm3/mol[7] | |||
| Thermal conductivity | 0.204 W/(m·K) at 25 °C[8] | ||
Refractive index (nD)
|
1.3817 (20 °C)[3] | ||
| Viscosity | 0.63 cP at 25 °C[8] | ||
| 3.46[9] | |||
| Explosive data | |||
| Shock sensitivity | Low | ||
| Friction sensitivity | Low | ||
| Detonation velocity | 6400 m/s | ||
| Thermochemistry[10] | |||
Heat capacity (C)
|
106.6 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
171.8 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
-112.6 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
-14.4 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, health hazard | ||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H203, H226, H301, H331, H351 | |||
| P210, P261, P280, P304+P340, P312, P370+P378, P403+P233 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 35[9] °C (95 °F; 308 K) | ||
| 418[9] °C (784 °F; 691 K) | |||
| Explosive limits | 7–22%[9] | ||
Threshold limit value (TLV)
|
20 ppm[9] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
940 mg/kg (oral, rat) 950 mg/kg (oral, mouse)[11] | ||
LDLo (lowest published)
|
750 mg/kg (rabbit, oral) 125 mg/kg (dog, oral)[11] | ||
LCLo (lowest published)
|
7087 ppm (mouse, 2 h) 1000 ppm (monkey) 2500 ppm (rabbit, 12 h) 5000 ppm (rabbit, 6 h)[11] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 100 ppm (250 mg/m3)[2] | ||
REL (Recommended)
|
none[2] | ||
IDLH (Immediate danger)
|
750 ppm[2] | ||
| Related compounds | |||
Related nitro compounds
|
nitroethane | ||
Related compounds
|
methyl nitrite methyl nitrate | ||
| Supplementary data page | |||
| Nitromethane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
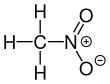
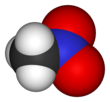
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings.[12] Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 662. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0457". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d e Haynes, p. 3.414
- ^ Haynes, p. 6.69
- ^ Haynes, p. 5.94
- ^ Reich, Hans. "Bordwell pKa table: "Nitroalkanes"". University of Wisconsin Chemistry Department. Retrieved 27 January 2022.
- ^ Haynes, p. 3.576
- ^ a b Haynes, p. 6.231
- ^ a b c d e Haynes, p. 15.19
- ^ Haynes, p. 5.20
- ^ a b c "Nitromethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Cite error: The named reference
Markofskywas invoked but never defined (see the help page).