
Back تحمض المحيطات Arabic Окисляване на океана Bulgarian মহাসাগরের অম্লতা বৃদ্ধি Bengali/Bangla Acidificació de l'oceà Catalan Okyselování oceánů Czech Havenes forsuring Danish Versauerung der Meere German Οξίνιση των ωκεανών Greek Oceana acidiĝo Esperanto Acidificación del océano Spanish
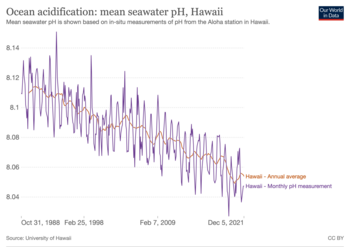
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.[2] Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO2) levels exceeding 422 ppm (as of 2024[update]).[3] CO2 from the atmosphere is absorbed by the oceans. This chemical reaction produces carbonic acid (H2CO3) which dissociates into a bicarbonate ion (HCO−3) and a hydrogen ion (H+). The presence of free hydrogen ions (H+) lowers the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.[4]
A change in pH by 0.1 represents a 26% increase in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH units is equivalent to a tenfold change in hydrogen ion concentration). Sea-surface pH and carbonate saturation states vary depending on ocean depth and location. Colder and higher latitude waters are capable of absorbing more CO2. This can cause acidity to rise, lowering the pH and carbonate saturation levels in these areas. There are several other factors that influence the atmosphere-ocean CO2 exchange, and thus local ocean acidification. These include ocean currents and upwelling zones, proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture.[5][6][7]
A lower ocean pH has a range of potentially harmful effects for marine organisms. Scientists have observed for example reduced calcification, lowered immune responses, and reduced energy for basic functions such as reproduction.[8] Ocean acidification can impact marine ecosystems that provide food and livelihoods for many people. About one billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. Ongoing acidification of the oceans may therefore threaten food chains linked with the oceans.[9][10]
The only solution that would address the root cause of ocean acidification is to reduce carbon dioxide emissions. This is one of the main objectives of climate change mitigation measures. The removal of carbon dioxide from the atmosphere would also help to reverse ocean acidification. In addition, there are some specific ocean-based mitigation methods, for example ocean alkalinity enhancement and enhanced weathering. These strategies are under investigation, but generally have a low technology readiness level and many risks.[11][12][13]
Ocean acidification has happened before in Earth's geologic history.[14] The resulting ecological collapse in the oceans had long-lasting effects on the global carbon cycle and climate.
- ^ Ritchie, Roser, Mispy, Ortiz-Ospina. "SDG 14 – Measuring progress towards the Sustainable Development Goals Archived 22 January 2022 at the Wayback Machine." SDG-Tracker.org, website (2018).
- ^ Terhaar, Jens; Frölicher, Thomas L.; Joos, Fortunat (2023). "Ocean acidification in emission-driven temperature stabilization scenarios: the role of TCRE and non-CO2 greenhouse gases". Environmental Research Letters. 18 (2): 024033. Bibcode:2023ERL....18b4033T. doi:10.1088/1748-9326/acaf91. ISSN 1748-9326. S2CID 255431338.
Figure 1f
- ^ Oxygen, Pro (21 September 2024). "Earth's CO2 Home Page". Retrieved 21 September 2024.
- ^ Cite error: The named reference
raven05was invoked but never defined (see the help page). - ^ Cite error: The named reference
Jian2019was invoked but never defined (see the help page). - ^ Zhang, Y.; Yamamoto-Kawai, M.; Williams, W.J. (16 February 2020). "Two Decades of Ocean Acidification in the Surface Waters of the Beaufort Gyre, Arctic Ocean: Effects of Sea Ice Melt and Retreat From 1997–2016". Geophysical Research Letters. 47 (3). doi:10.1029/2019GL086421. S2CID 214271838.
- ^ Beaupré-Laperrière, Alexis; Mucci, Alfonso; Thomas, Helmuth (31 July 2020). "The recent state and variability of the carbonate system of the Canadian Arctic Archipelago and adjacent basins in the context of ocean acidification". Biogeosciences. 17 (14): 3923–3942. Bibcode:2020BGeo...17.3923B. doi:10.5194/bg-17-3923-2020. S2CID 221369828.
- ^ Anthony, K. R. N.; Kline, D. I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. (11 November 2008). "Ocean acidification causes bleaching and productivity loss in coral reef builders". Proceedings of the National Academy of Sciences. 105 (45): 17442–17446. Bibcode:2008PNAS..10517442A. doi:10.1073/pnas.0804478105. PMC 2580748. PMID 18988740.
- ^ Dean, Cornelia (30 January 2009). "Rising Acidity Is Threatening Food Web of Oceans, Science Panel Says". New York Times.
- ^ Service, Robert E. (13 July 2012). "Rising Acidity Brings an Ocean of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- ^ IPCC (2022) Chapter 12: Cross sectoral perspectives
- ^ Archived 13 October 2022 at the Wayback Machine in Climate Change 2022: Mitigation of Climate ChangeContribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change]
- ^ Archived 2 August 2022 at the Wayback Machine, Cambridge University Press, Cambridge, United Kingdom and New York, NY, US: 12–36
- ^ Bärbel Hönisch; Andy Ridgwell; Daniela N Schmidt; et al. (2 March 2012). "The geological record of ocean acidification". Science. 335 (6072): 1058–63. Bibcode:2012Sci...335.1058H. doi:10.1126/SCIENCE.1208277. ISSN 0036-8075. PMID 22383840. Wikidata Q28261134.