
Back أورنيثين Arabic اورنیتین AZB অরনিথিন Bengali/Bangla Ornitin BS Ornitina Catalan Ornitin Czech Ornithin German Ορνιθίνη Greek Ornitina Spanish اورنیتین Persian
| |

| |
| Names | |
|---|---|
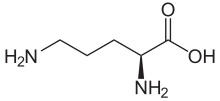
| IUPAC name
L-Ornithine
| |
| Other names
(+)-(S)-2,5-Diaminovaleric acid
(+)-(S)-2,5-Diaminopentanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.665 |
| EC Number |
|
| KEGG | |
| MeSH | Ornithine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C5H12N2O2 | |
| Molar mass | 132.16 g/mol |
| Melting point | 140 °C (284 °F; 413 K) |
| soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | 1.94 |
Chiral rotation ([α]D)
|
+11.5 (H2O, c = 6.5) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.[2]
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-408. ISBN 0-8493-0462-8.
- ^ Sivashanmugam M (February 2017). "Ornithine and its role in metabolic diseases: An appraisal". Biomed Pharmacother. 86: 185–194. doi:10.1016/j.biopha.2016.12.024. PMID 27978498.