
Back Pantoteensuur Afrikaans فيتامين بي5 Arabic پانتوتنیک اسید AZB Пантотенова киселина Bulgarian Vitamin B5 BS Àcid pantotènic Catalan Kyselina pantothenová Czech Asid pantothenig Welsh Pantothensäure German ވިޓަމިން ބީ5 DV
| |

| |
| Names | |
|---|---|
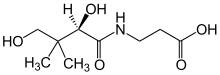
| Preferred IUPAC name
3-[(2R)-2,4-Dihydroxy-3,3-dimethylbutanamido]propanoic acid | |
| Systematic IUPAC name
3-[(2R)-(2,4-Dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1727062, 1727064 (R) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.061 |
| EC Number |
|
| KEGG | |
| MeSH | Pantothenic+Acid |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H17NO5 | |
| Molar mass | 219.237 g·mol−1 |
| Appearance | Yellow oil Colorless crystals (Ca2+ salt) |
| Odor | Odorless |
| Density | 1.266 g/cm3 1.32 g/cm3 (Ca2+ salt)[1] |
| Melting point | 183.833 °C (362.899 °F; 456.983 K) 196–200 °C (385–392 °F; 469–473 K) decomposes (Ca2+ salt)[1][3][5] |
| Very soluble[2] 2.11 g/mL (Ca2+ salt)[1] | |
| Solubility | Very soluble in C6H6, ether[2] Ca2+ salt: Slightly soluble in alcohol, CHCl3[3] |
| log P | −1.416[4] |
| Acidity (pKa) | 4.41[5] |
| Basicity (pKb) | 9.698 |
Chiral rotation ([α]D)
|
+37.5° +24.3° (Ca2+ salt)[5] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 10 mg/g (Ca2+ salt)[3] |
| Related compounds | |
Related alkanoic acids
|
Arginine Hopantenic acid 4-(γ-Glutamylamino)butanoic acid |
Related compounds
|
Panthenol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pantothenic acid (vitamin B5) is a B vitamin and an essential nutrient.[6] All animals need pantothenic acid in order to synthesize coenzyme A (CoA), which is essential for cellular energy production and for the synthesis and degradation of proteins, carbohydrates, and fats.[6][7]
Pantothenic acid is the combination of pantoic acid and β-alanine. Its name comes from the Greek πάντοθεν pantothen, meaning "from everywhere", because pantothenic acid, at least in small amounts, is in almost all foods.[6][8][7] Deficiency of pantothenic acid is very rare in humans.[6][7] In dietary supplements and animal feed, the form commonly used is calcium pantothenate, because chemically it is more stable, and hence makes for longer product shelf-life, than sodium pantothenate and free pantothenic acid.[1]
- ^ a b c d "Scientific Opinion on the safety and efficacy of pantothenic acid (calcium D-pantothenate and D-panthenol) as a feed additive for all animal species based on a dossier submitted by Lohmann Animal Health". EFSA Journal. 9 (11). Parma, Italy: European Food Safety Authority: 2409. 2011. doi:10.2903/j.efsa.2011.2409.
- ^ a b Lide DR, ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b c "Calcium D-pantothenate". CHEMICALLAND21, AroKor Holdings Inc. Retrieved 5 September 2014.
- ^ "MSDS of D-pantothenic acid" (PDF). Human Metabolome Database. Retrieved 5 September 2014.
- ^ a b c Leenheer AP, Lambert WE, Bocxlaer JF, eds. (2000). Modern Chromatographic Analysis of Vitamins: Revised And Expanded. Chromatographic Science. Vol. 84 (3rd ed.). Marcel Dekker. p. 533. ISBN 978-0-203-90962-1.
- ^ a b c d "Pantothenic acid: Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 3 June 2020. Retrieved 27 November 2020.
- ^ a b c "Pantothenic acid". Linus Pauling Institute at Oregon State University. Micronutrient Information Center. 1 July 2015. Retrieved 27 November 2020.
- ^ Cite error: The named reference
usdawas invoked but never defined (see the help page).
