
Back باروكسيتين Arabic پاروکستین AZB Paroxetina Catalan Parocsetin Welsh Paroxetin German Παροξετίνη Greek Paroxetina Spanish Paroxetina Basque پاروکستین Persian Paroksetiini Finnish
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paxil, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (By mouth) |
| Drug class | Selective serotonin reuptake inhibitor (SSRI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extensively absorbed from the GI tract, but extensive first-pass metabolism in the liver[3][4][5][6] |
| Protein binding | 93–95%[3][4][5] |
| Metabolism | Extensive, liver (mostly CYP2D6-mediated)[3][4][5] |
| Elimination half-life | 21 hours[3][4][5] |
| Excretion | Kidney (64%; 2% unchanged and 62% as metabolites), faecal (36%; <1% unchanged)[3][4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.112.096 |
| Chemical and physical data | |
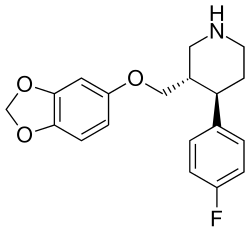
| Formula | C19H20FNO3 |
| Molar mass | 329.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Paroxetine, sold under the brand name Paxil among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[7] It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, post-traumatic stress disorder, generalized anxiety disorder, and premenstrual dysphoric disorder.[7] It has also been used in the treatment of premature ejaculation and hot flashes due to menopause.[7][8] It is taken orally (by mouth).[7]
Common side effects include drowsiness, dry mouth, loss of appetite, sweating, trouble sleeping, and sexual dysfunction.[7] Serious side effects may include suicidal thoughts in those under the age of 25, serotonin syndrome, and mania.[7] While the rate of side effects appears similar compared to other SSRIs and SNRIs, antidepressant discontinuation syndromes may occur more often.[9][10] Use in pregnancy is not recommended, while use during breastfeeding is relatively safe.[11] It is believed to work by blocking the reuptake of the chemical serotonin by neurons in the brain.[7]
Paroxetine was approved for medical use in the United States in 1992 and initially sold by GlaxoSmithKline.[7][12] It is on the World Health Organization's List of Essential Medicines.[13] It is available as a generic medication.[14] In 2022, it was the 92nd most commonly prescribed medication in the United States, with more than 7 million prescriptions.[15][16] In 2018, it was in the top 10 of most prescribed antidepressants in the United States.[17]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ a b c d e Sandoz Pty Ltd (18 January 2012). "Product Information Paroxetine Sandoz 20Mg Film-Coated Tablets" (PDF). Therapeutic Goods Administration. Archived from the original on 4 September 2015. Retrieved 22 November 2013.
- ^ a b c d e Mylan Institutional Inc. (January 2012). "Paroxetine (paroxetine hydrochloride hemihydrate) tablet, film coated". DailyMed. U.S. National Library of Medicine. Archived from the original on 23 October 2013. Retrieved 22 November 2013.
- ^ a b c d e Sandoz Limited (21 March 2013). "Paroxetine 20 mg Tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Datapharm Ltd. Archived from the original on 3 December 2013. Retrieved 22 November 2013.
- ^ "Paxil, Paxil CR (paroxetine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 10 November 2015. Retrieved 22 November 2013.
- ^ a b c d e f g h "Paroxetine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ Fischer A (28 June 2013). "FDA approves the first non-hormonal treatment for hot flashes associated with menopause" (Press release). Food and Drug Administration. Archived from the original on 18 January 2017.
- ^ Hosenbocus S, Chahal R (February 2011). "SSRIs and SNRIs: A review of the Discontinuation Syndrome in Children and Adolescents". Journal of the Canadian Academy of Child and Adolescent Psychiatry. 20 (1): 60–67. PMC 3024727. PMID 21286371.
- ^ Pae CU, Patkar AA (February 2007). "Paroxetine: current status in psychiatry". Expert Review of Neurotherapeutics. 7 (2): 107–120. doi:10.1586/14737175.7.2.107. PMID 17286545. S2CID 34636522.
- ^ "Paroxetine Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 3 December 2018. Retrieved 3 March 2019.
- ^ Food and Drug Administration (2011). Approved Drug Products with Therapeutic Equivalence Evaluations – FDA Orange Book 31st Edition (2011): FDA Orange Book 31st Edition (2011). DrugPatentWatch.com. p. 344. ISBN 9781934899816. Archived from the original on 6 March 2019. Retrieved 4 March 2019.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 363. ISBN 9780857113382.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Paroxetine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Grohol JM (15 December 2019). "Top 25 Psychiatric Medications for 2018". psychcentral.com. Archived from the original on 25 October 2020. Retrieved 26 September 2020.