
Back حمض الفيتيك Arabic فیتیک اسید AZB Fitinska kiselina BS Àcid fític Catalan Kyselina fytová Czech Fytin Danish Phytinsäure German Φυτικό οξύ Greek Ácido fítico Spanish Azido fitiko Basque
| |
 | |

| |
| Names | |
|---|---|
| IUPAC name
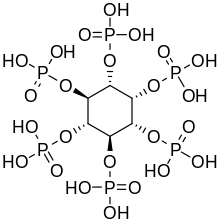
(1R,2S,3r,4R,5S,6s)-cyclohexane-1,2,3,4,5,6-hexayl hexakis[dihydrogen (phosphate)]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.369 |
| E number | E391 (antioxidants, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H18O24P6 | |
| Molar mass | 660.029 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phytic acid is a six-fold dihydrogenphosphate ester of inositol (specifically, of the myo isomer), also called inositol hexaphosphate, inositol hexakisphosphate (IP6) or inositol polyphosphate. At physiological pH, the phosphates are partially ionized, resulting in the phytate anion.
The (myo) phytate anion is a colorless species that has significant nutritional role as the principal storage form of phosphorus in many plant tissues, especially bran and seeds. It is also present in many legumes, cereals, and grains. Phytic acid and phytate have a strong binding affinity to the dietary minerals calcium, iron, and zinc, inhibiting their absorption in the small intestine.[1]
The lower inositol polyphosphates are inositol esters with less than six phosphates, such as inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3). These occur in nature as catabolites of phytic acid.
- ^ Schlemmer, U.; Frølich, W.; Prieto, R. M.; Grases, F. (2009). "Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis" (PDF). Molecular Nutrition & Food Research. 53 (Suppl 2): S330–75. doi:10.1002/mnfr.200900099. PMID 19774556.