
Back تعديل ما بعد الترجمة Arabic Posttranslacijske modifikacije BS Modificació posttraduccional Catalan Posttranslační modifikace Czech Posttranslationel modifikation Danish Posttranslationale Modifikation German Modificación postraduccional Spanish Translatsioonijärgne modifikatsioon Estonian پیرایش پساترجمهای Persian Modification post-traductionnelle French
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.
Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini.[1] They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. [2] Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability as well as serving regulatory functions. Attachment of lipid molecules, known as lipidation, often targets a protein or part of a protein attached to the cell membrane.
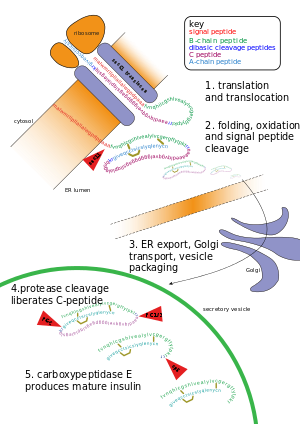
Other forms of post-translational modification consist of cleaving peptide bonds, as in processing a propeptide to a mature form or removing the initiator methionine residue. The formation of disulfide bonds from cysteine residues may also be referred to as a post-translational modification.[3] For instance, the peptide hormone insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds.
Some types of post-translational modification are consequences of oxidative stress. Carbonylation is one example that targets the modified protein for degradation and can result in the formation of protein aggregates.[4][5] Specific amino acid modifications can be used as biomarkers indicating oxidative damage.[6]
Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine forms of lysine, arginine, and histidine; the thiolate anion of cysteine; the carboxylates of aspartate and glutamate; and the N- and C-termini. In addition, although the amide of asparagine is a weak nucleophile, it can serve as an attachment point for glycans. Rarer modifications can occur at oxidized methionines and at some methylene groups in side chains.[7]
Post-translational modification of proteins can be experimentally detected by a variety of techniques, including mass spectrometry, Eastern blotting, and Western blotting. Additional methods are provided in the #External links section.
- ^ Pratt, Charlotte W.; Voet, Judith G.; Voet, Donald (2006). Fundamentals of Biochemistry: Life at the Molecular Level (2nd ed.). Hoboken, NJ: Wiley. ISBN 9780471214953. OCLC 1280801548. Archived from the original on 13 July 2012.
- ^ Khoury GA, Baliban RC, Floudas CA (September 2011). "Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database". Scientific Reports. 1: 90. Bibcode:2011NatSR...1E..90K. doi:10.1038/srep00090. PMC 3201773. PMID 22034591.
- ^ Lodish H, Berk A, Zipursky SL, et al. (2000). "17.6, Post-Translational Modifications and Quality Control in the Rough ER". Molecular Cell Biology (4th ed.). New York: W. H. Freeman. ISBN 978-0-7167-3136-8.
- ^ Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006). "Protein carbonylation, cellular dysfunction, and disease progression". Journal of Cellular and Molecular Medicine. 10 (2): 389–406. doi:10.1111/j.1582-4934.2006.tb00407.x. PMC 3933129. PMID 16796807.
- ^ Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (August 2008). "Oxidative stress and covalent modification of protein with bioactive aldehydes". The Journal of Biological Chemistry. 283 (32): 21837–41. doi:10.1074/jbc.R700019200. PMC 2494933. PMID 18445586.
- ^ Gianazza E, Crawford J, Miller I (July 2007). "Detecting oxidative post-translational modifications in proteins". Amino Acids. 33 (1): 51–6. doi:10.1007/s00726-006-0410-2. PMID 17021655. S2CID 23819101.
- ^ Walsh, Christopher T. (2006). Posttranslational modification of proteins : expanding nature's inventory. Englewood: Roberts and Co. Publ. ISBN 9780974707730. : 12–14