
Back بيكبريتات البوتاسيوم Arabic Kalium hidrosulfat Azerbaijani بیسولفات پوتاسیوم AZB Hydrogensíran draselný Czech Kaliumhydrogensulfat German Kalia bisulfato Esperanto Bisulfato de potasio Spanish پتاسیم بیسولفات Persian Kaliumvetysulfaatti Finnish Hydrogénosulfate de potassium French
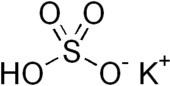
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium hydrogen sulfate
| |
| Other names
Potassium acid sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.722 |
| EC Number |
|
| E number | E515(ii) (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2509 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| KHSO4 | |
| Molar mass | 136.169 g/mol |
| Appearance | colorless solid |
| Odor | odorless |
| Density | 2.245 g/cm3 |
| Melting point | 197 °C (387 °F; 470 K) |
| Boiling point | 300 °C (572 °F; 573 K) (decomposes to form potassium pyrosulfate and water) |
| 36.6 g/100 mL (0 °C) 49 g/100 mL (20 °C) 121.6 g/100 mL (100 °C) | |
| Solubility | soluble in acetone, ethanol. |
| −49.8·10−6 cm3/mol | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-1163.3 kJ/mol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H335 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P363, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2340 mg*kg−1 |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Potassium sulfate Sodium bisulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium bisulfate (potassium bisulphate) is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.