Back Kaliumchloraat Afrikaans كلورات البوتاسيوم Arabic Kalium xlorat Azerbaijani پوتاسیوم کولورات AZB Калиев хлорат Bulgarian Clorat de potassi Catalan Chlorečnan draselný Czech Бертолле тăварĕ CV Kaliumklorat Danish Kaliumchlorat German
| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
Potassium chlorate(V), Potcrate, Berthollet salt
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.021.173 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1485 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
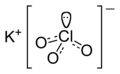
| KClO3 | |||
| Molar mass | 122.55 g mol−1 | ||
| Appearance | white crystals or powder | ||
| Density | 2.32 g/cm3 | ||
| Melting point | 356 °C (673 °F; 629 K) | ||
| Boiling point | 400 °C (752 °F; 673 K) decomposes[1] | ||
| 3.13 g/100 mL (0 °C) 4.46 g/100 mL (10 °C) 8.15 g/100 mL (25 °C) 13.21 g/100 mL (40 °C) 53.51 g/100 mL (100 °C) 183 g/100 g (190 °C) 2930 g/100 g (330 °C)[2] | |||
| Solubility | soluble in glycerol negligible in acetone and liquid ammonia[1] | ||
| Solubility in glycerol | 1 g/100 g (20 °C)[1] | ||
| −42.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.40835 | ||
| Structure | |||
| monoclinic | |||
| Thermochemistry | |||
Heat capacity (C)
|
100.25 J/mol·K[1] | ||
Std molar
entropy (S⦵298) |
142.97 J/mol·K[3][1] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−391.2 kJ/mol[3][1] | ||
Gibbs free energy (ΔfG⦵)
|
-289.9 kJ/mol[1] | ||
| Hazards | |||
| GHS labelling: | |||
   [4] [4]
| |||
| Danger | |||
| H271, H302, H332, H411[4] | |||
| P220, P273[4] | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1870 mg/kg (oral, rat)[5] | ||
| Safety data sheet (SDS) | ICSC 0548 | ||
| Related compounds | |||
Other anions
|
Potassium bromate Potassium iodate Potassium nitrate | ||
Other cations
|
Ammonium chlorate Sodium chlorate Barium chlorate | ||
Related compounds
|
Potassium chloride Potassium hypochlorite Potassium chlorite Potassium perchlorate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches.[6] In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used
- in fireworks, propellants and explosives,
- to prepare oxygen, both in the lab and in chemical oxygen generators,
- as a disinfectant, for example in dentifrices and medical mouthwashes,
- in agriculture as an herbicide.
- ^ a b c d e f g "potassium chlorate". Retrieved 9 July 2015.
- ^ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. Retrieved 2014-05-29.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ a b c "Potassium chlorate". Retrieved 14 February 2022.
- ^ Michael Chambers. "ChemIDplus - 3811-04-9 - VKJKEPKFPUWCAS-UHFFFAOYSA-M - Potassium chlorate - Similar structures search, synonyms, formulas, resource links, and other chemical information". Retrieved 9 July 2015.
- ^ Vogt, Helmut; Balej, Jan; Bennett, John E.; Wintzer, Peter; Sheikh, Saeed Akbar; Gallone, Patrizio (June 15, 2000). "Chlorine Oxides and Chlorine Oxygen Acids". In Ullmann (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag. doi:10.1002/14356007.a06_483. ISBN 9783527303854.