
Back Kaliumpermanganaat Afrikaans بيرمنغنات البوتاسيوم Arabic Kalium permanqanat Azerbaijani پوتاسیوم پرمنقنات AZB Калиев перманганат Bulgarian পটাশিয়াম পারম্যাঙ্গানেট Bengali/Bangla Kalij-permanganat BS Permanganat de potassi Catalan Manganistan draselný Czech Kaliumpermanganat Danish
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium manganate(VII)
| |
| Systematic IUPAC name
Potassium permanganate | |
| Other names
Chameleon mineral
Condy's crystals Permanganate of potash Hypermangan Purple potion powder | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.874 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1490 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
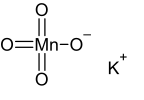
| KMnO4 | |
| Molar mass | 158.032 g·mol−1 |
| Appearance | Purplish-bronze-gray needles purple in solution[1] |
| Odor | odorless |
| Density | 2.7 g/cm3[2]: 4.83 |
| Melting point | 240 °C (464 °F; 513 K) (decomposes) |
| 76 g/L (25 °C)[2] 250 g/L (65 °C) | |
| Solubility | soluble in organic solvents; decomposes in alcohol |
| +20.0·10−6 cm3/mol[2]: 4.134 | |
Refractive index (nD)
|
1.59 |
| Structure[3] | |
| Orthorhombic, oP24 | |
| Pnma, No. 62 | |
a = 0.909 nm, b = 0.572 nm, c = 0.741 nm
| |
Formula units (Z)
|
4 |
| Thermochemistry | |
Heat capacity (C)
|
119.2 J/mol K |
Std molar
entropy (S⦵298) |
171.7 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−813.4 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-713.8 kJ/mol |
| Pharmacology | |
| D08AX06 (WHO) V03AB18 (WHO) | |
| Hazards | |
| GHS labelling: | |
  
| |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1090 mg/kg (oral, rat)[4] |
| Related compounds | |
Other anions
|
Potassium pertechnetate Potassium perrhenate |
Other cations
|
Sodium permanganate Ammonium permanganate Calcium permanganate Silver permanganate |
Related manganates
|
Potassium hypomanganate Potassium manganate |
Related compounds
|
Manganese heptoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Clinical data | |
|---|---|
| License data | |
| Identifiers | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.874 |
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and MnO−
4 ions to give an intensely pink to purple solution.
Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines.[5] In 2000, worldwide production was estimated at 30,000 tons.[5]
- ^ Burriel F, Lucena F, Arribas S, Hernández J (1985). Química Analítica Cualitativa [Qualitative Analytical Chemistry] (in Spanish). Ediciones Paraninfo, S.A. p. 688. ISBN 84-9732-140-5.
- ^ a b c Haynes WM, ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
- ^ Hocart R, Mathieu-Sicaud A (1945). "A stabilization factor in the polymorphism of ammonium nitrate". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences [Weekly Reports of the Sessions of the Academy of Sciences] (in French). 221: 261–263.
- ^ Chambers M. "Potassium permanganate [USP:JAN] – Similar structures search, synonyms, formulas, resource links, and other chemical information". ChemIDplus: A Toxnet database. U.S. National Library of Medicine. ChemIDplus – CAS: 7722-64-7 InChi: VZJVWSHVAAUDKD-UHFFFAOYSA-N. Archived from the original on 13 August 2014. Retrieved 9 May 2018.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
