
Back Sout (chemiese stof) Afrikaans Salze ALS Sal (quimica) AN लवण ANP ملح (كيمياء) Arabic লৱণ Assamese Sal (adobíu) AST Duz (kimya) Azerbaijani Тоҙҙар Bashkir Droskas BAT-SMG
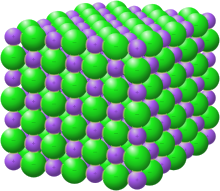
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions),[1] which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds.
The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (CH
3COO−
). Each ion can be either monatomic (termed simple ion), such as fluoride (F−), and sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as sulfate (SO2−
4), and ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, for example sodium hydroxide.
Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures when solid.
Salts composed of small ions typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions become mobile. Some salts have large cations, large anions, or both. In terms of their properties, such species often are more similar to organic compounds.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "salt". doi:10.1351/goldbook.S05447