
Back سيميبريفير Arabic Simeprevir Catalan Simeprevir Czech Simeprevir Spanish سیمپرویر Persian Simepreviiri Finnish Siméprévir French Simeprevir ID Simeprevir Italian ସିମେପ୍ରେଭିର OR
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪˈmɛprəvɪər/ si-MEP-rə-veer |
| Trade names | Olysio, Sovriad, Galexos, others |
| Other names | TMC435; TMC435350 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614013 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 62% (under fed conditions) |
| Protein binding | >99.9% |
| Metabolism | Liver (CYP3A, CYP2C8, CYP2C19) |
| Elimination half-life | 10–13 hours (HCV-uninfected subjects), 41 hours (HCV-infected subjects) |
| Excretion | Feces (91%), urine (<1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.933 |
| Chemical and physical data | |
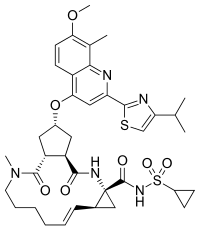
| Formula | C38H47N5O7S2 |
| Molar mass | 749.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C.[2] It is specifically used for hepatitis C genotype 1 and 4.[2] Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa.[2] Cure rates are in 80s to 90s percent.[3][4][5] It may be used in those who also have HIV/AIDS.[2] It is taken by mouth once daily for typically 12 weeks.[2]
Common side effects include feeling tired, headache, rash, itchiness, and sensitivity to sunlight.[2] In those with previous hepatitis B infection, active disease may recur.[2] It is not recommended in those with significant liver problems.[2] During pregnancy when used with ribavirin it may cause harm to the baby while when used with sofosbuvir its safety is unclear.[2][6] Simeprevir is a HCV protease inhibitor.[2]
Simeprevir was developed by Medivir AB and Janssen Pharmaceutica.[7] It was approved for medical use in the United States in 2013.[8] It was removed from the World Health Organization's List of Essential Medicines in 2019.[9][10] It is not available as a generic medication as of 2015[update].[6]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ a b c d e f g h i j "Simeprevir". The American Society of Health-System Pharmacists. Archived from the original on 1 December 2016. Retrieved 30 November 2016.
- ^ "Initial treatment of HCV infection". www.hcvguidelines.org. October 2016. Archived from the original on 7 December 2016. Retrieved 1 December 2016.
- ^ Majumdar A, Kitson MT, Roberts SK (June 2016). "Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis". Alimentary Pharmacology & Therapeutics. 43 (12): 1276–92. doi:10.1111/apt.13633. PMID 27087015.
- ^ Brochot E, Helle F, François C, Castelain S, Capron D, Nguyen-Khac E, Duverlie G (April 2015). "Which therapeutic option for hepatitis C virus genotype 1?". Scandinavian Journal of Gastroenterology. 50 (4): 470–8. doi:10.3109/00365521.2014.978364. PMID 25396710. S2CID 34382861.
- ^ a b Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 80. ISBN 9781284057560.
- ^ Grubbs RH, O'Leary DJ (2015). Handbook of Metathesis, Volume 2: Applications in Organic Synthesis. John Wiley & Sons. p. 699. ISBN 9783527694020.
- ^ Dugum M, O'Shea R (March 2014). "Hepatitis C virus: here comes all-oral treatment". Cleveland Clinic Journal of Medicine. 81 (3): 159–72. doi:10.3949/ccjm.81a.13155. PMID 24591471. S2CID 37838853.
- ^ World Health Organization (2019). Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. hdl:10665/325773. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2019). The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. hdl:10665/330668. ISBN 9789241210300. ISSN 0512-3054. WHO technical report series;1021.