
Back Sterol Afrikaans ستيرول Arabic Стэрыны Byelorussian স্টেরল Bengali/Bangla Sterol BS Esterol Catalan Steroly Czech Sterol Danish Sterine German Στερόλη Greek
| |
| Names | |
|---|---|
| IUPAC name
2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17-hexadecahydro-1H-cyclopenta[a]phenanthren-3-ol
| |
| Other names
Hexadecahydro-3H-cyclopenta[a]phenanthrene-3-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C17H28O | |
| Molar mass | 248.410 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
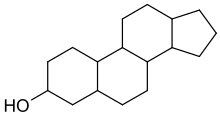
Sterol is an organic compound[1] with formula C
17H
28O, whose molecule is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). [2][3] The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones.
While technically alcohols, sterols are classified by biochemists as lipids (fats in the broader sense of the term).

- ^ "sterol (CHEBI:15889)". www.ebi.ac.uk. Retrieved 2023-11-04.
- ^ Wei JH, Yin X, Welander PV (24 June 2016). "Sterol Synthesis in Diverse Bacteria". Frontiers in Microbiology. 7: 990. doi:10.3389/fmicb.2016.00990. PMC 4919349. PMID 27446030.
- ^ Hoshino Y, Gaucher EA (June 2021). "Evolution of bacterial steroid biosynthesis and its impact on eukaryogenesis". Proceedings of the National Academy of Sciences of the United States of America. 118 (25): e2101276118. Bibcode:2021PNAS..11801276H. doi:10.1073/pnas.2101276118. PMC 8237579. PMID 34131078.