
Back انزياح ستوكس Arabic Stokesův posuv Czech Stokes-Verschiebung German Stokesi nihe Estonian Déplacement de Stokes French היסט סטוקס HE Stokesov pomak Croatian Ստոքսի կանոն Armenian Spostamento di Stokes Italian ストークスシフト Japanese
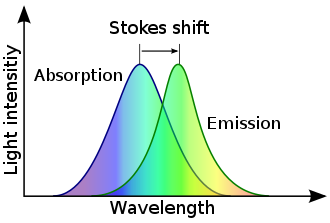
Stokes shift is the difference (in energy, wavenumber or frequency units) between positions of the band maxima of the absorption and emission spectra (fluorescence and Raman being two examples) of the same electronic transition.[1] It is named after Irish physicist George Gabriel Stokes.[2][3][4]

When a system (be it a molecule or atom) absorbs a photon, it gains energy and enters an excited state. The system can relax by emitting a photon. The Stokes shift occurs when the energy of the emitted photon is lower than that of the absorbed photon, representing the difference in energy of the two photons.
The Stokes shift is primarily the result of two phenomena: vibrational relaxation or dissipation and solvent reorganization. A fluorophore is a part of a molecule with a dipole moment that exhibits fluorescence. When a fluorophore enters an excited state, its dipole moment changes, but surrounding solvent molecules cannot adjust so quickly. Only after vibrational relaxation do their dipole moments realign.[5]
Stokes shifts are given in wavelength units, but this is less meaningful than energy, wavenumber or frequency units because it depends on the absorption wavelength. For instance, a 50 nm Stokes shift from absorption at 300 nm is larger in terms of energy than a 50 nm Stokes shift from absorption at 600 nm.
- ^ Gispert, J. R. (2008). Coordination Chemistry. Wiley-VCH. p. 483. ISBN 978-3-527-31802-5.[permanent dead link]
- ^ Albani, J. R. (2004). Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies. Elsevier. p. 58. ISBN 0-444-51449-X.
- ^ Lakowicz, J. R. 1983. Principles of Fluorescence Spectroscopy, Plenum Press, New York. ISBN 0-387-31278-1.
- ^ Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York. ISBN 0-8247-8350-6.
- ^ "Fluorescence Microscopy - Basic Concepts in Fluorescence | Olympus LS". www.olympus-lifescience.com. Retrieved 2024-04-14.