 | |
| Clinical data | |
|---|---|
| Trade names | Ospolot |
| Other names | Sulthiame (AAN AU), sulthiame (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (oral) |
| Protein binding | 29% |
| Metabolism | Hepatic secretion |
| Elimination half-life | 24 hours |
| Excretion | Fecal (10%) and renal (90%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.465 |
| Chemical and physical data | |
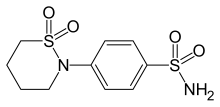
| Formula | C10H14N2O4S2 |
| Molar mass | 290.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sultiame (or sulthiame) is a sulfonamide and inhibitor of the enzyme carbonic anhydrase. It is used as an anticonvulsant and in recent studies showed promise in reducing sleep disordered breathing and other symptoms of obstructive sleep apnea (OSA).
