
Back مستقبل خلية تي Arabic Receptor dels limfòcits T Catalan T-buněčný receptor Czech T-Zell-Rezeptor German Receptor de linfocitos T Spanish T-rakkude retseptorid Estonian گیرنده لنفوسیت تی Persian Antigeenireseptori Finnish Récepteur des lymphocytes T French Receptor de células T Galician
| TCR complex | |
|---|---|
 | |
| Identifiers | |
| Symbol | TCR |
| OPM superfamily | 166 |
| Membranome | 26 |
| T-cell receptor alpha locus | |
|---|---|
| Identifiers | |
| Symbol | TRA |
| Alt. symbols | TCRA, TRA@ |
| NCBI gene | 6955 |
| HGNC | 12027 |
| OMIM | 186880 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor beta locus | |
|---|---|
| Identifiers | |
| Symbol | TRB |
| Alt. symbols | TCRB, TRB@ |
| NCBI gene | 6957 |
| HGNC | 12155 |
| OMIM | 186930 |
| Other data | |
| Locus | Chr. 7 q34 |
| T-cell receptor delta locus | |
|---|---|
| Identifiers | |
| Symbol | TRD |
| Alt. symbols | TCRD, TRD@, TCRDV1 |
| NCBI gene | 6964 |
| HGNC | 12252 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor gamma locus | |
|---|---|
| Identifiers | |
| Symbol | TRG |
| Alt. symbols | TCRG, TRG@ |
| NCBI gene | 6965 |
| HGNC | 12271 |
| Other data | |
| Locus | Chr. 7 p14 |
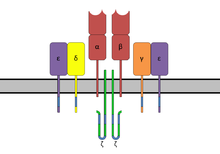
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes,[1] that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.[2]
The TCR is composed of two different protein chains (that is, it is a heterodimer). In humans, in 95% of T cells the TCR consists of an alpha (α) chain and a beta (β) chain (encoded by TRA and TRB, respectively), whereas in 5% of T cells the TCR consists of gamma and delta (γ/δ) chains (encoded by TRG and TRD, respectively). This ratio changes during ontogeny and in diseased states (such as leukemia). It also differs between species. Orthologues of the 4 loci have been mapped in various species.[3][4] Each locus can produce a variety of polypeptides with constant and variable regions.[3]
When the TCR engages with antigenic peptide and MHC (peptide/MHC), the T lymphocyte is activated through signal transduction, that is, a series of biochemical events mediated by associated enzymes, co-receptors, specialized adaptor molecules, and activated or released transcription factors. Based on the initial receptor triggering mechanism, the TCR belongs to the family of non-catalytic tyrosine-phosphorylated receptors (NTRs).[5]
- ^ Kindt TJ, Goldsby RA, Osborne BA, Kuby J (2007). Kuby immunology. Macmillan. pp. 223–. ISBN 978-1-4292-0211-4. Retrieved 28 November 2010.
- ^ Sewell AK (September 2012). "Why must T cells be cross-reactive?". Nature Reviews. Immunology. 12 (9): 669–77. doi:10.1038/nri3279. PMC 7097784. PMID 22918468.
- ^ a b Glusman G, Rowen L, Lee I, Boysen C, Roach JC, Smit AF, et al. (September 2001). "Comparative genomics of the human and mouse T cell receptor loci". Immunity. 15 (3): 337–49. doi:10.1016/s1074-7613(01)00200-x. PMID 11567625.
- ^ Deakin JE, Parra ZE, Graves JA, Miller RD (2006). "Physical mapping of T cell receptor loci (TRA@, TRB@, TRD@ and TRG@) in the opossum (Monodelphis domestica)". Cytogenetic and Genome Research. 112 (3–4): 342K. doi:10.1159/000089901. PMID 16484802.
- ^ Dushek O, Goyette J, van der Merwe PA (November 2012). "Non-catalytic tyrosine-phosphorylated receptors". Immunological Reviews. 250 (1): 258–76. doi:10.1111/imr.12008. PMID 23046135. S2CID 1549902.