
Back حمض الطرطريك Arabic Mezoçaxır turşusu Azerbaijani اسید تارتاریک AZB Винена киселина Bulgarian Vinska kiselina BS Àcid tàrtric Catalan Kyselina vinná Czech Asid tartarig Welsh Vinsyre Danish Weinsäure German

| |
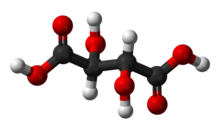 Ball-and-stick model of L-(+)-tartaric acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydroxybutanedioic acid | |
| Other names
Tartaric acid
2,3-Dihydroxysuccinic acid Threaric acid Racemic acid Uvic acid Paratartaric acid Winestone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.903 |
| E number | E334 (antioxidants, ...) |
| KEGG | |
| MeSH | tartaric+acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O6 (basic formula) HO2CCH(OH)CH(OH)CO2H (structural formula) | |
| Molar mass | 150.087 g/mol |
| Appearance | White powder |
| Density | 1.737 g/cm3 (R,R- and S,S-) 1.79 g/cm3 (racemate) 1.886 g/cm3 (meso) |
| Melting point | 169, 172 °C (R,R- and S,S-) 206 °C (racemate) 165-6 °C (meso) |
| |
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.89, pKa2= 4.40 meso 25 °C: pKa1= 3.22, pKa2= 4.85 |
| Conjugate base | Bitartrate |
| −67.5·10−6 cm3/mol | |
| Hazards | |
| GHS labelling:[3] | |

| |
| Danger | |
| H318 | |
| P280, P305+P351+P338+P310 | |
| Related compounds | |
Other cations
|
Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
Related carboxylic acids
|
Butyric acid Succinic acid Dimercaptosuccinic acid Malic acid Maleic acid Fumaric acid |
Related compounds
|
2,3-Butanediol Cichoric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus.[1] Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.
- ^ a b Tartaric Acid – Compound Summary, PubChem.
- ^ Dawson, R.M.C. et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ GHS: Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health